Advertisements
Advertisements
Question
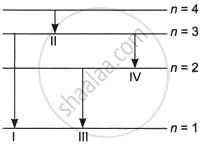
The diagram shows the four energy levels of an electron in the Bohr model of the hydrogen atom. Identify the transition in which the emitted photon will have the highest energy.
Options
I
II
III
IV
Solution
I
Explanation:
The correct scale is used to create the energy diagram, which displays the various energy levels.
The largest energy difference occurs during the change from state 3 to state 1. The first jump would be the most energetic and provide the strongest photon emission.
APPEARS IN
RELATED QUESTIONS
Given the ground state energy E0 = - 13.6 eV and Bohr radius a0 = 0.53 Å. Find out how the de Broglie wavelength associated with the electron orbiting in the ground state would change when it jumps into the first excited state.
A 12.5 eV electron beam is used to bombard gaseous hydrogen at room temperature. Upto which energy level the hydrogen atoms would be excited? Calculate the wavelengths of the first member of Lyman and first member of Balmer series.
A difference of 2.3 eV separates two energy levels in an atom. What is the frequency of radiation emitted when the atom makes a transition from the upper level to the lower level?
The total energy of an electron in the first excited state of the hydrogen atom is about −3.4 eV.
What is the kinetic energy of the electron in this state?
Obtain the first Bohr’s radius and the ground state energy of a muonic hydrogen atom [i.e., an atom in which a negatively charged muon (μ−) of mass about 207 me orbits around a proton].
What are means by pair annihilation? Write a balanced equation for the same.
Which transition corresponds to emission of radiation of maximum wavelength?
A Carnot engine absorbs 1000 J of heat energy from a reservoir at 127°C and rejects 600 J of heat energy during each cycle. The efficiency of the engine and temperature of the sink will be:
Energy of an electron at infinity from nucleus is ______.
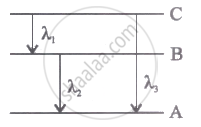
Energy levels A, B, C of acertain atom corresponding to increasing value of energy, i.e., EA< E8 < Ee. If λ1, λ2 and λ3 are the wavelength of radiations corresponding to the transitions C to B, B to A and C to A respectively, which of the following statements is correct?