Advertisements
Advertisements
Question
Which one of the following would feel attraction when placed in magnetic field: Co2+, Ag+, Ti4+, Zn2+
Solution
Co2+ has three unpaired electrons so it would be paramagnetic in nature, hence Co2+ ion would be attracted to magnetic field.
APPEARS IN
RELATED QUESTIONS
What is ferromagnetism?
Write the type of magnetism observed when the magnetic moments are oppositely aligned and cancel out each other.
Explain the following with suitable examples: Ferrimagnetism
Explain the following with suitable examples: Antiferromagnetism
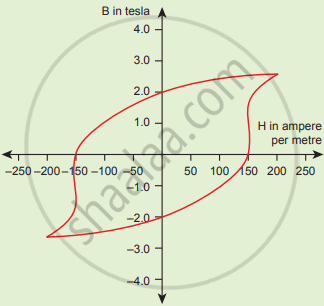
The BH curve for a ferromagnetic material is shown in the figure. The material is placed inside a long solenoid which contains 1000 turns/cm. The current that should be passed in the solenonid to demagnetize the ferromagnet completely is
Example of ferromagnetic substance is ____________.
When heated to high temperature, ferromagnetic substance changes to ____________.
Which of the following arrangements shows schematic alignment of magnetic moments of antiferromagnetic substances?
A ferromagnetic substance becomes a permanent magnet when it is placed in a magnetic field becuase ______.
Given below are two statements labelled as Assertion (A) and Reason (R).
Assertion: Magnetic moment values of actinides are lesser than the theoretically predicted values.
Reason: Actinide elements are strongly paramagnetic.
Select the most appropriate answer from the options given below:
