Advertisements
Advertisements
Question
Why does our palm feel cold when we put some acetone or petrol or perfume on it?
Short Answer
Solution
- When we put some acetone or petrol or perfume on our palm, it evaporates.
- During evaporation, particles of the liquid absorb energy from the surrounding or the surface of the palm to compensate for the loss of energy, making the surroundings cool.
- Hence, our palm feels cold when we put some acetone or petrol or perfume on it.
shaalaa.com
Is there an error in this question or solution?
APPEARS IN
RELATED QUESTIONS
Why is cooling produced on evaporation of a liquid?
Define the following terms Condensation
How does the water kept in an earthen pot (matka) become cold during summer ?
The amount of vapour in 1 cu.m. of air shows the ______.
Why is the air in a region dry?
Can you suggest a method to collect water from sea water?
Look at Fig. 1.3 and suggest in which of the vessels A,B, C or D the rate of evaporation will be the highest? Explain.
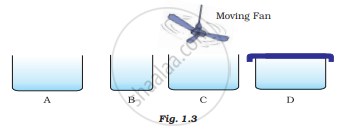
The rate of evaporation ______ with rising temperature.
Evaporation is a fast process and occurs only at the surface of the liquid.
The rate of evaporation is more when the surface area is greater.
