Advertisements
Advertisements
Question
Write a balanced chemical equation of the reaction which takes place.
Solution
CuO(s) + 2HCl(aq) → CuCl2(aq) + H2O(l)
APPEARS IN
RELATED QUESTIONS
With the help of labelled diagrams, describe an activity to show that acids produce ions only in aqueous solutions.
Name the gas evolved when zinc granules are treated/heated with:
hydrochloric acid solution
What ions are present in the solutions of following substances? (write the symbols only)
Sodium hydroxide
What is the chemical name of bleaching powder?
Choose the correct alternative and rewrite the following sentence.
Phenolphthalein is ___________ type of indicator.
A student added 10 g of calcium carbonate in a rigid container, secured it tightly and started to heat it. After some time, an increase in pressure was observed, the pressure reading was then noted at intervals of 5 mins and plotted against time, in a graph as shown below. During which time interval did maximum decomposition take place?
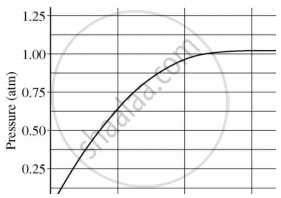
Which of the following substance will not give carbon dioxide on treatment with dilute acid?
Which of the following statements is not correct?
A sulphate salt of Group 2 element of the Periodic Table is a white, soft substance which can be moulded into different shapes by making its dough. When this compound is left in open for some time, it becomes a solid mass and cannot be used for moulding purposes. Identify the sulphate salt. Why does it show such a behaviour? Give the reaction involved.
Consider the following salt:
\[\ce{ZCO3}\]
What would be the change in colour in blue litmus if \[\ce{ZCO3}\] is added to it and Z is potassium?
