Advertisements
Advertisements
Question
Write down the word equation or balanced equation for the action of concentrated nitric acid on copper.
Solution
Balanced equation:
\[\ce{Cu + 4HNO3 ->[\Delta] Cu(NO3)2 + 2NO2 + 2H2O}\]
word equation :
\[\ce{Copper + nitric acid ->[\Delta] copper nitrate + nitrogen dioxide + water}\]
APPEARS IN
RELATED QUESTIONS
Write balanced equations for action of warm water on magnesium nitride
What is : aqua regia
During thunderstorm, rain water contains nitric acid. Explain with reactions.
In the preparation of nitric acid from KNO3 concentrated hydrochloric acid is not used in place of concentrated sulphuric acid. Explain why?
Give reasons for the following:
In the laboratory preparation of nitric acid, the mixture of concentrated sulphuric acid and sodium nitrate should not be heated very strongly above 200°C.
Write the balanced equation for the following:
Action of heat on AgNO3
Write the equation to show the reaction between the following:
Between copper and concentrated nitric acid.
Fill in the blank using the appropriate words given below:
Hot, concentrated nitric add reacts with sulphur to form ______.
From the formulae listed below, Choose, one, corresponding to the salt having the given description:
AgCl, CuCO3, CuSO4.5H2O, KNO3, NaCl, NaHSO4, Pb(NO3)2, ZnCO3, ZnSO4, 7H2O.
On heating this, salt changes from green to black.
The diagram given below is a representation of the Industrial preparation of Nitric acid by Ostwald’s process. With respect to the process answer the following questions:
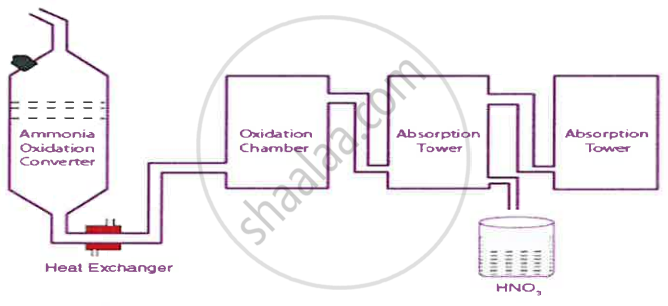
- Write the temperature and the catalyst required during the catalytic oxidation of ammonia.
- Give balanced chemical equation for the reaction occurring duringthe conversion of nitrogen dioxide to nitric acid.
