Advertisements
Advertisements
Question
Write Lewis structure of the following compounds and show formal charge on atom.
\[\ce{H2SO4}\]
Solution
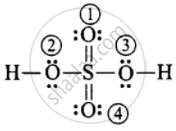
F.C. on O (1) and (4) `-> 6 - 4 - 1/2 (4) = 0`
F.C. on O (2) and (3) `-> 6 - 4 - 1/2 (4) = 0`
F.C. on H `-> 1 - 0 - 1/2 (2) = 0`
F.C. on S `-> 6 - 0 - 1/2 (12) = 0`
APPEARS IN
RELATED QUESTIONS
Draw the Lewis structures for the following molecule and ion:
H2S
Draw the Lewis structures for the given molecule and ion:
SiCl4
Draw the Lewis structures for the given molecule and ion:
BeF2
Draw the Lewis structures for the given molecule and ion:
`"CO"_3^(2-)`
Draw the Lewis structures for the following molecule and ion:
HCOOH
According to Lewis theory, a base is any species which ____________.
Write Lewis structure of the following compounds and show formal charge on atom.
\[\ce{HNO3}\]
Write Lewis structure of the following compounds and show formal charge on atom.
\[\ce{NO2}\]
Represent diagrammatically the bond moments and the resultant dipole moment in \[\ce{CO2, NF3}\] and \[\ce{CHCl3}\].
Which of the following species contains equal number of σ– and π–bonds?
