Advertisements
Advertisements
Question
Write Lewis structure of the following compounds and show formal charge on atom.
\[\ce{HNO3}\]
Solution
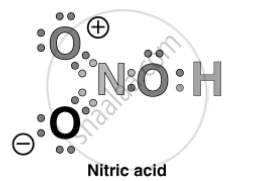
Formal charge on an atom in a Lewis structure = [Total number of valence electrons in free atom] – [Total number of non-bonding (lone pairs) electrons]
= `- 1/2` [Total number of bonding or shared electrons]
APPEARS IN
RELATED QUESTIONS
Write Lewis dot symbols for atoms of the following elements: Mg, Na, B, O, N, Br.
Draw the Lewis structures for the given molecule and ion:
SiCl4
Draw the Lewis structures for the given molecule and ion:
BeF2
Draw the Lewis structures for the given molecule and ion:
`"CO"_3^(2-)`
Draw the Lewis structures for the following molecule and ion:
HCOOH
Explain why BeH2 molecule has a zero dipole moment although the Be–H bonds are polar.
According to Lewis theory, a base is any species which ____________.
Write Lewis structure of the following compounds and show formal charge on atom.
\[\ce{NO2}\]
Write Lewis structure of the following compounds and show formal charge on atom.
\[\ce{H2SO4}\]
Represent diagrammatically the bond moments and the resultant dipole moment in \[\ce{CO2, NF3}\] and \[\ce{CHCl3}\].
