Advertisements
Advertisements
Question
Write Lewis dot symbols for atoms of the following elements: Mg, Na, B, O, N, Br.
Solution 1
Mg: There are two valence electrons in Mg atom. Hence, the Lewis dot symbol for Mg is: ![]()
Na: There is only one valence electron in an atom of sodium. Hence, the Lewis dot structure is: ![]()
B: There are 3 valence electrons in Boron atom. Hence, the Lewis dot structure is: 
O: There are six valence electrons in an atom of oxygen. Hence, the Lewis dot structure is: 
N: There are five valence electrons in an atom of nitrogen. Hence, the Lewis dot structure is: 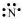
Br: There are seven valence electrons in bromine. Hence, the Lewis dot structure is: 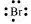
Solution 2
`""_12Mg` = 2,8,2 ∴ Lewis symbol = 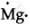
`""_11Mg` = 2,8,1 ∴ Lewis symbol = 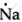
`""_5B` = 2,3 ∴ Lewis symbol = 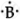
`""_8O` =2,6 ∴ Lewis symbol = 
`""_7N` = 2 , 5 ∴ Lewis symbol = 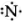
`""_35Br`= 2,8,18,7 ∴ Lewis symbol = 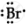
APPEARS IN
RELATED QUESTIONS
Draw the Lewis structures for the following molecule and ion:
H2S
Draw the Lewis structures for the given molecule and ion:
SiCl4
Draw the Lewis structures for the given molecule and ion:
BeF2
Draw the Lewis structures for the given molecule and ion:
`"CO"_3^(2-)`
Draw the Lewis structures for the following molecule and ion:
HCOOH
Explain why BeH2 molecule has a zero dipole moment although the Be–H bonds are polar.
According to Lewis theory, a base is any species which ____________.
Write Lewis structure of the following compounds and show formal charge on atom.
\[\ce{HNO3}\]
Write Lewis structure of the following compounds and show formal charge on atom.
\[\ce{NO2}\]
Write Lewis structure of the following compounds and show formal charge on atom.
\[\ce{H2SO4}\]
Represent diagrammatically the bond moments and the resultant dipole moment in \[\ce{CO2, NF3}\] and \[\ce{CHCl3}\].
Which of the following species contains equal number of σ– and π–bonds?
