Advertisements
Advertisements
Question
Write the formula of the product formed when the element A (atomic number 19) combines with the element B (atomic number 17). Draw its electronic dot structure. What is the nature of the bond formed?
Solution
Element A has atomic number 19 so, it is potassium. Element B has atomic number 17 so, it is chlorine. As the valency of potassium is 1 and the valency of chlorine is also 1, the formula of the compound formed would be KCl or AB.
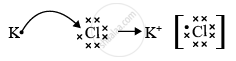
The nature of the bond between potassium and chlorine in KCl is an ionic bond.
APPEARS IN
RELATED QUESTIONS
Which is greater in size Fe2+ or Fe3+?
Study the radius of the element given below and answer the following questions.
| elements | K | Na | Rb | Cs | Li |
| Atomic radius (pm) | 231 | 186 | 244 | 262 | 151 |
a) Which of the above elements have the smallest atom?
b) In which group of the modern periodic table the above element are belongs?
c) What is the periodic trend observed in the variation of atomic radii down a group?
The following questions refer to the Periodic Table.
What happens to the atomic size of elements moving from top to bottom of a group?
The metals of Group 2 from top to bottom are Be, Mg, Ca, Sr and Ba. Which one of these elements will form ions most readily and why?
Arrange the following as per the instruction given in the bracket
Na, K, Li (Increasing atomic size)
Atomic radius is expressed in the unit _______.
Carbon belongs to the second period and Group 14. Silicon belongs to the third period and Group 14. If the atomic number of carbon is 6, the atomic number of silicon is ______
Which of the following is the correct order of atomic size?
Elements have been arranged in the following sequence on the basis of their increasing atomic masses.
| F, | Na, | Mg, | Al, | Si, | P, | S, | Cl, | Ar, | K |
- Pick two sets of elements which have similar properties.
- The given sequence represents which law of classification of elements?
Arrange the following as per instruction given in the bracket.
Mg, Cl, Na, S, Si (decreasing order of atomic size)
