Advertisements
Advertisements
Question
You must have seen tarnished copper vessels being cleaned with lemon or tamarind juice. Explain why these sour substances are effective in cleaning the vessels.
Solution
Copper reacts with moist carbon dioxide in the air to form copper carbonate, which causes a copper vessel to lose its shiny brown surface and form a green layer of copper carbonate. The citric acid present in lemon or tamarind neutralises the base copper carbonate and dissolves the layer. That is why tarnished copper vessels are cleaned with lemon or tamarind juice to give the surface its characteristic lustre.
\[\ce{2Cu + \underset{(Present in air)}{H2O + CO2 + O2} -> \underset{(green)}{\underset{Basic copper carbonate}{CuCO3.CU(OH)2}}}\]
APPEARS IN
RELATED QUESTIONS
_________ solution is blue in colour.
(a) CuSO4
(b) FeSO4
(c) ZnSO4
(d) Al2(SO4)3
Why is sodium kept immersed in kerosene oil?
Food cans are coated with tin and not with zinc because ______.
Differentiate between metal and non-metal on the basis of their chemical properties.
The reaction of an iron nail with copper sulphate solution is ........... reaction.
(a) Combination
(b) Displacement
(c) Double displacement
(d) Decomposition
What name is given to those metal oxides which show basic as well as acidic behaviour?
Name the metal which has been placed just below copper in the reactivity series.
Complete and balance the following equation:
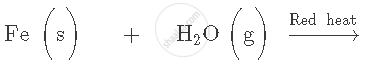
Complete and balance the following equation:
Cu (NO3)2 (aq) + Zn(s) →
Write the chemical equation of the reaction which takes place when iron reacts with dilute sulphuric acid. What happens when the gas produced is ignited with a burning matchstick?
Write the equation for the reaction of Magnesium with dilute hydrochloric acid.
Name the products formed. Also indicate the physical states of all the substances involved.
The least reactive metal among the following is:
(a) sodium
(b) silver
(c) copper
(d) lead
An element X forms two oxides XO and XO2. The oxide XO is neutral but XO2 is acidic in nature. The element X is most likely to be:
(a) sulphur
(b) carbon
(c) calcium
(d) hydrogen
An element E forms an oxide E2O. An aqueous solution of E2O turns red litmus paper blue.
(a) What is the nature of the oxide E2O?
(b) State whether element E is a metal or a non-metal.
(c) Give one example of an element like E.
CuSO4(aq) + Fe(s) → FeSO4(aq) + Cu(s)
FeSO4(aq) + Zn(s) → ZnSO4(aq) + Fe(s)
On the basis of the above reactions, indicate which is most reactive and which is least reactive metal out of zinc, copper and iron.
Name a metal which does not react with cold as well as hot water but reacts with steam.
What is the characteristic of the electronic configuration of noble gases?
Generally, metals are solid in nature. Which one of the following metals is found in liquid state at room temperature?
The substance that will be flattened on beating with a hammer is
Tungsten wire is used in electric bulbs.
