English Medium
Academic Year: 2021-2022
Date & Time: 10th May 2022, 10:30 am
Duration: 2h
Advertisements
General Instructions :
Read the following instructions carefully and strictly follow them:
-
This Question paper contains 15 questions. All questions are compulsory.
-
This question paper is divided into three Sections viz. Sections A, B, and C.
-
Section-A—Question number 1 to 7 are short answer type questions. Each question carries two marks.
-
Section-B—Question number 8 to 13 are also short answer type questions. Each question carries three marks.
-
Section-C—Question number 14 to 15 are case-based questions. Each question carries four marks.
-
Internal choices have been provided in some questions. Only one of the alternatives has to be attempted.
"Carbon prefers to share its valence electrons with other atoms of carbon or with atoms of other elements rather than gaining or losing the valence electrons in order to attain noble gas configuration." Give reasons to justify this statement.
Chapter: [0.03] Metals and Non Metals [0.04] Carbon and its Compounds
The atomic number of an element 'X' is 11.
- Write the electronic configuration of X and find its valency.
- Write the formula and nature of its oxide.
Chapter: [0.05] Periodic Classification of Elements
Give reason.
Placenta is extremely essential for foetal development.
Chapter: [0.07] How do Organisms Reproduce?
Give reason:
Uterine lining becomes thick and spongy after fertilisation.
Chapter: [0.07] How do Organisms Reproduce?
Name the reproductive and non-reproductive parts of bread mould (Rhizopus).
Chapter: [0.07] How do Organisms Reproduce?
List two advantages of vegetative propagation.
Chapter: [0.07] How do Organisms Reproduce?
In the following figure showing a germinating gram seed, name the parts labelled as A, B and C:
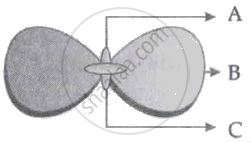
Why is Part 'B' considered to be important during germination?
Chapter: [0.08] Heredity
A part of modern periodic table is given below. On its basis, answer the following questions:
| Group No. → | 1 | 2 | 13 | 14 | 15 | 16 | 17 | 18 |
| Period ↓ | ||||||||
| 2 | A | B | ||||||
| 3 | E | D | F | C |
Write the molecular formula of the compound formed by the combination:
A and F
Chapter: [0.05] Periodic Classification of Elements
A part of modern periodic table is given below. On its basis, answer the following questions:
| Group No. → | 1 | 2 | 13 | 14 | 15 | 16 | 17 | 18 |
| Period ↓ | ||||||||
| 2 | A | B | ||||||
| 3 | E | D | F | C |
Write the molecular formula of the compound formed by the combination:
E and B
Chapter: [0.05] Periodic Classification of Elements
Advertisements
A part of modern periodic table is given below. On its basis, answer the following questions:
| Group No. → | 1 | 2 | 13 | 14 | 15 | 16 | 17 | 18 |
| Period ↓ | ||||||||
| 2 | A | B | ||||||
| 3 | E | D | F | C |
Which of the element is a Noble gas?
Chapter: [0.05] Periodic Classification of Elements
A part of modern periodic table is given below. On its basis, answer the following questions:
| Group No. → | 1 | 2 | 13 | 14 | 15 | 16 | 17 | 18 |
| Period ↓ | ||||||||
| 2 | A | B | ||||||
| 3 | E | D | F | C |
Which of the element is a Metalloid?
Chapter: [0.05] Periodic Classification of Elements
Write the chemical formula of two consecutive homologous of organic compounds having functional group - OH.
What happens to the (i) boiling point and (ii) solubility of organic compounds of a homologous series as the molecular mass increases?
Chapter: [0.04] Carbon and its Compounds
State Newland’s Law of Octaves.
Chapter: [0.05] Periodic Classification of Elements
With an example, explain Dobereiner's Triads.
Chapter: [0.05] Periodic Classification of Elements
List two limitations of Newlands' Law of Octaves.
Chapter: [0.05] Periodic Classification of Elements
What were the limitations of Dobereiner’s classification?
Chapter: [0.05] Periodic Classification of Elements
Consider the following organic compounds:
| (i) \[\begin{array}{cc} \ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\ |\phantom{....}|\phantom{....}|\\ \ce{H - C - C - C = O}\\ |\phantom{....}|\phantom{.....}\\ \ce{H}\phantom{....}\ce{H}\phantom{.....} \end{array}\] |
(ii) \[\begin{array}{cc} \phantom{....}\ce{H}\phantom{...}\ce{H}\phantom{....}\\ \phantom{....}|\phantom{....}|\phantom{....}\\ \ce{H - C - C = O}\\|\phantom{.....}\\ \ce{H}\phantom{.....}\end{array}\] |
- Name the functional group present in these compounds.
- Write the general formula for the compounds of this functional group.
- State the relationship between these compounds and draw the structure of any other compound having a similar functional group.
Chapter: [0.04] Carbon and its Compounds
Draw the electron dot structure for ethyne.
Chapter: [0.04] Carbon and its Compounds
List two differences between the properties exhibited by covalent compounds and ionic compounds.
Chapter: [0.03] Metals and Non Metals [0.04] Carbon and its Compounds
Advertisements
In the given circuit determine the value of:
- Total resistance of the circuit
- Current flowing through the ammeter.

Chapter: [0.11] Electricity
A green stemmed tomato plant denoted by (GG) is crossed with a tomato plant with purple stem denoted by (gg).
- What colour of the stem would you expect in their F1 progeny?
- In what ratio would you find the green and purple coloured stem in plants of F2 progeny?
- What conclusion can be drawn for the above observations?
Chapter: [0.08] Heredity
On what factors does the resistance of a conductor depend?
Chapter: [0.11] Electricity
The resistance of a wire of 0.01 cm radius is 10 Ω. If the resistivity of the wire is 50 × 10-8 Ω, find the length of this wire.
Chapter: [0.11] Electricity
What is the meaning of electric power of an electrical device? Write its SI unit.
Chapter: [0.11] Electricity
An electric kettle of 2 kW is used for 2h. Calculate the energy consumed in
- kilowatt hour and
- joules.
Chapter: [0.11] Electricity
We do not clean ponds or lakes, but an aquarium needs to be cleaned. Why?
Chapter: [0.13] Our Environment
Why is ozone layer getting depleted at the higher levels of the atmosphere? Mention one harmful effect caused by its depletion.
Chapter: [0.13] Our Environment
Mendel blended his knowledge of science and mathematics to keep count of the individuals exhibiting a particular trait in each generation. He observed a number of contrasting visible characters controlled in pea plants in a field. He conducted many experiments to arrive at the laws of inheritance.
- What do the F1 progeny of tall plants with round seeds and short plants with wrinkled seeds look like?
- Name the recessive traits in the above case.
- Mention the type of the new combinations of plants obtained in F2 progeny along with their ratio, if F1 progeny was allowed to self-pollinate.
OR
If 1600 plants were obtained in F2 progeny, write the number of plants having traits:
- Tall with round seeds.
- Short with wrinkled seeds.
Write the conclusion of the above experiment.
Chapter: [0.08] Heredity
A student was asked to perform an experiment to study the force on a current carrying conductor in a magnetic field. He took a small aluminium rod AB, a strong horse shoe magnet, some connecting wires, a battery and a switch and connected them as shown. He observed that on passing current, the rod gets displaced. On reversing the direction of current, the direction of displacement also gets reversed. On the basis of your understanding of this phenomenon, answer the following questions:
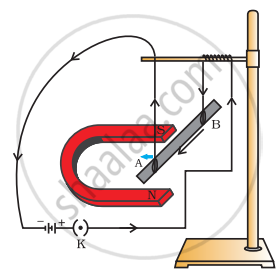
- Why does the rod get displaced on passing current through it?
- State the rule that determines the direction of the force on the conductor AB.
-
- If the U-shaped magnet is held vertically and the aluminium rod is suspended horizontally with its end B towards due north, then on passing current through the rod from B to A as shown, in which direction will the rod be displaced?
- Name any two devices that use current carrying conductors and magnetic fields.
OR
Draw the pattern of magnetic field lines produced around a current carrying straight conductor held vertically on a horizontal cardboard. Indicate the direction of the field lines as well as the direction of current flowing through the conductor.
Chapter: [0.12] Magnetic Effects of Electric Current
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CBSE previous year question papers Class 10 Science with solutions 2021 - 2022
Previous year Question paper for CBSE Class 10 Science-2022 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Science, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CBSE Class 10.
How CBSE Class 10 Question Paper solutions Help Students ?
• Question paper solutions for Science will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
