Advertisements
Advertisements
प्रश्न
A colourless substance ‘A’ \[\ce{(C6H7N)}\] is sparingly soluble in water and gives a water soluble compound ‘B’ on treating with mineral acid. On reacting with \[\ce{CHCl3}\] and alcoholic potash ‘A’ produces an obnoxious smell due to the formation of compound ‘C’. Reaction of ‘A’ with benzenesulphonyl chloride gives compound ‘D’ which is soluble in alkali. With \[\ce{NaNO2}\] and \[\ce{HCl}\], ‘A’ forms compound ‘E’ which reacts with phenol in alkaline medium to give an orange dye ‘F’. Identify compounds ‘A’ to ‘F’.
उत्तर
(i)
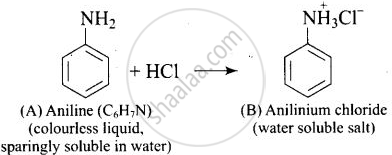
(ii)
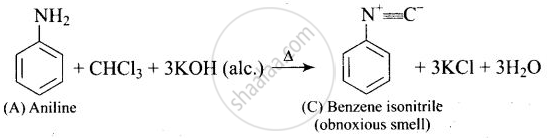
(iii)
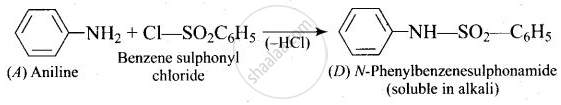
(iv)
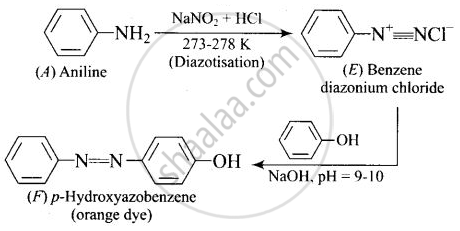
APPEARS IN
संबंधित प्रश्न
Illustrate the following reaction giving suitable example in each case: Diazotisation
Why does acetylation of –NH2 group of aniline reduce its activating effect?
Predict the product of reaction of aniline with bromine in non-polar solvent such as \[\ce{CS2}\].
Why is aniline soluble in aqueous HCl?
Match the compounds given in Column I with the items given in Column II.
| Column I | Column II | ||
| (i) | Benzene sulphonyl chloride | (a) | Zwitter ion |
| (ii) | Sulphanilic acid | (b) | Hinsberg reagent |
| (iii) | Alkyl diazonium salts | (c) | Dyes |
| (iv) | Aryl diazonium salts | (d) | Conversion to alcohols |
In order to distinguish between C2H5NHz and C6H5NHz, which of the following reagents is useful?
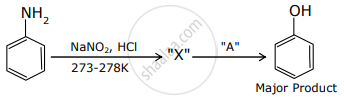
Major Product In the above chemical reaction, intermediate "X" and reagent/condition "A" are:
Aniline dissolved in dilute HCl is reacted with sodium nitrate at 0 °C. This solution was added dropwise to a solution containing equimolar mixture of aniline and phenol in dil. HCl. The structure of the major product is:
Identify A and B for the following reaction:

What are polymers?
How will the following be converted? (Give chemical equation)
Aniline to benzene diazonium chloride.
