Advertisements
Advertisements
प्रश्न
A colourless substance ‘A’ \[\ce{(C6H7N)}\] is sparingly soluble in water and gives a water soluble compound ‘B’ on treating with mineral acid. On reacting with \[\ce{CHCl3}\] and alcoholic potash ‘A’ produces an obnoxious smell due to the formation of compound ‘C’. Reaction of ‘A’ with benzenesulphonyl chloride gives compound ‘D’ which is soluble in alkali. With \[\ce{NaNO2}\] and \[\ce{HCl}\], ‘A’ forms compound ‘E’ which reacts with phenol in alkaline medium to give an orange dye ‘F’. Identify compounds ‘A’ to ‘F’.
उत्तर
(i)
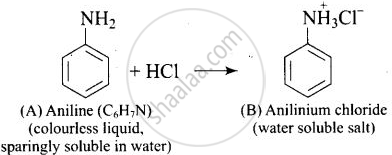
(ii)
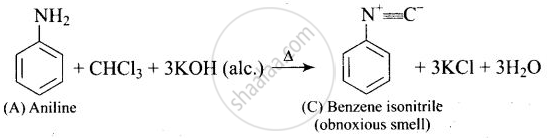
(iii)
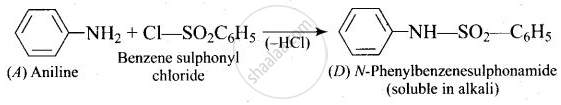
(iv)
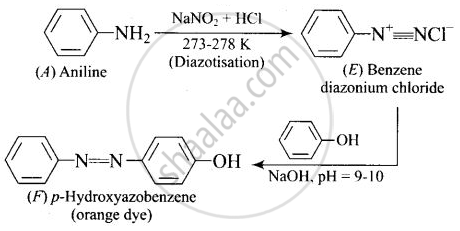
APPEARS IN
संबंधित प्रश्न
Why is benzene diazonium chloride not stored and is used immediately after its preparation?
Why is aniline soluble in aqueous HCl?
How will you carry out the following conversions?
toluene `->` p-toluidine
Match the compounds given in Column I with the items given in Column II.
| Column I | Column II | ||
| (i) | Benzene sulphonyl chloride | (a) | Zwitter ion |
| (ii) | Sulphanilic acid | (b) | Hinsberg reagent |
| (iii) | Alkyl diazonium salts | (c) | Dyes |
| (iv) | Aryl diazonium salts | (d) | Conversion to alcohols |
In order to distinguish between C2H5NHz and C6H5NHz, which of the following reagents is useful?
Aniline when treated with cone. HNO3 gives
Which of the following is the most stable diazonium salt?
Benzene diazonium chloride is a ______.
Identify A and B for the following reaction:

What are polymers?
How will the following be converted? (Give chemical equation)
Aniline to benzene diazonium chloride.
