Advertisements
Advertisements
प्रश्न
Assertion (A): Pent- 1- ene and pent- 2- ene are position isomers.
Reason (R): Position isomers differ in the position of functional group or a substituent.
विकल्प
Both A and R are correct and R is the correct explanation of A.
Both A and R are correct but R is not the correct explanation of A.
Both A and R are not correct.
A is not correct but R is correct.
उत्तर
Both A and R are correct and R is the correct explanation of A.
Explanation:
When two or more compounds differ in the position of substituent atom or functional group on the carbon skeleton, they are known as position isomers and this phenomenon is termed as position isomerism. Pent-2-ene and pent-l-ene are position isomers because they differ in the position of the double bond.
APPEARS IN
संबंधित प्रश्न
Write IUPAC name of the product obtained by the ozonolysis of the following compound:
3,4-Dimethyl-hept-3-ene
Write IUPAC name of the product obtained by the ozonolysis of the following compound:
2-Ethylbut-1-ene
Draw the cis and trans structures of hex-2-ene. Which isomer will have higher b.p. and why?
What is the relationship between the members of following pairs of structures? Are they structural or geometrical isomers or resonance contributors?
\[\begin{array}{cc}
\ce{D}\phantom{......}\ce{H}\\
\backslash\phantom{......}/\\
\ce{C = C}\\
\phantom{...}/\phantom{......}\backslash\phantom{...}\\\ce{H}\phantom{.......}\ce{D}
\end{array}\]
\[\begin{array}{cc}
\ce{D}\phantom{......}\ce{D}\\
\backslash\phantom{......}/\\
\ce{C = C}\\
\phantom{...}/\phantom{......}\backslash\phantom{...}\\\ce{H}\phantom{.......}\ce{H}\end{array}\]
Find out the type of isomerism exhibited by the following pair.
\[\begin{array}{cc}
\ce{CH3 - CH - CH2 - CH3 and CH3 - CH2 - O - CH2 - CH3}\\|\phantom{...........................................}\\
\ce{OH}\phantom{.........................................}\end{array}\]
Find out the type of isomerism exhibited by the following pair.
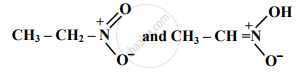
Choose the correct option.
Which type of isomerism is possible in CH3 CHCHCH3?
What type(s) of isomerism is(are) shown by [Co(NH3)4Br2]Cl?
Consider structures I to VII and answer the question:
| I. | CH3 – CH2 – CH2 – CH2 – OH |
| II. | \[\begin{array}{cc} \ce{CH3 - CH2 - CH - CH3}\\ \phantom{.....}|\\ \phantom{.......}\ce{OH} \end{array}\] |
| III. | \[\begin{array}{cc} \phantom{...}\ce{CH3}\\ \phantom{}|\\ \ce{CH3 - C - CH3}\\ \phantom{}|\\ \phantom{..}\ce{OH} \end{array}\] |
| IV. | \[\begin{array}{cc} \ce{CH3 - CH - CH2 - OH}\\ |\phantom{........}\\ \ce{CH3}\phantom{......} \end{array}\] |
| V. | CH3 – CH2 – O – CH2 – CH3 |
| VI. | CH3 – O – CH2 – CH2 – CH3 |
| VII. | \[\begin{array}{cc} \ce{CH3 - O - CH - CH3}\\ \phantom{...}|\\ \phantom{......}\ce{CH3} \end{array}\] |
Identify the pairs of compounds that represents chain isomerism.
Compounds with same molecular formula but differing in their structures are said to be structural isomers. What type of structural isomerism is shown by
CH3 – S – CH2 – CH2 – CH3
And
\[\begin{array}{cc}
\phantom{.....................}\ce{CH3}\\
\phantom{................}/\\
\phantom{}\ce{CH3 - S - CH}\\
\phantom{...............}\backslash\\
\phantom{....................}\ce{CH3}
\end{array}\]
The molecules having dipole moment are:
(i) 2,2-Dimethylpropane
(ii) trans-Pent-2-ene
(iii) cis-Hex-3-ene
(iv) 2, 2, 3, 3 - Tetramethylbutane.
Which of the following does NOT exhibit geometrical isomerism?
The compound which shows metamerism is ______
Acetamide is isomer of ______.
Which one of the following pairs are called position isomers?
How many structural isomers possible of the molecular formula C3H6O (excluding enol form)?
The number of acyclic structural isomers (including geometrical isomers) for pentene are ______.
Compound with molecular formula C3H6O can show ______.
The number of geometrical isomers from [Co(NH3)3(NO2)3] is ______.
