Advertisements
Advertisements
प्रश्न
Based on the group valency of elements state the formula lot the following giving justification for each :-
(i) Oxides of 1st group elements,
(ii) Halides of the elements of group 13, and
(iii) Compounds formed when an element of group 2 combines with, an element of group 16.
उत्तर
(i) Valency of group 1 elements: 1
Valency of oxygen: 2
Oxides of group 1 elements:
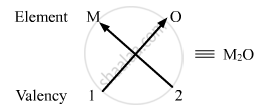
Formula of the oxides of group 1 is M2O, where M is the group 1 element and O is oxygen.
(ii) Valency of group 13 elements: 3
Valency of halogens: 1
Halides of group 13 elements:
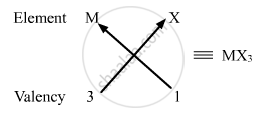
Formula of the halides of group 13 is MX3, where M is the group 13 element and X is halogen.
(iii) Valency of group 2 elements: 2
Valency of group 16 elements: 2
Compounds of group 2 and group 16 elements:
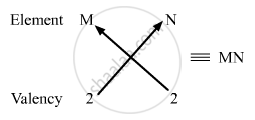
Formula of the compounds of group 2 and 16 is MN, where M is the group 2 element and N is the group 16 element.
APPEARS IN
संबंधित प्रश्न
Fill in the blank in the following statement:
In going across a period (right to left) in periodic table, the atomic size of the atom ...............
An element A has atomic number 14. To which period does this element belong and how many elements are there in this period.
Calculate the valency of element X whose atomic number is 9.
How could the atomic radius of a noble gas be compared with the other elements in a period?
Give the number of the group and the period of elements having three shells with three electrons in valence shell.
Choose the correct answer from the choice given:
An element A belonging to Period 3 and Group II will have
Arrange the following as per instruction given in the bracket.
Li, K, Na, H ( decreasing order of their potential ionisation )
Name and state the following with reference to the elements of the first three periods of the periodic table.
Metalloid in Period 3.
What is meant by a Group in the Periodic Table?
Name or state following with reference to the element of the first three periods of the periodic table.
A covalent compound formed between an element in period 1 and a halogen.
