Advertisements
Advertisements
प्रश्न
Based on the group valency of elements state the formula lot the following giving justification for each :-
(i) Oxides of 1st group elements,
(ii) Halides of the elements of group 13, and
(iii) Compounds formed when an element of group 2 combines with, an element of group 16.
उत्तर
(i) Valency of group 1 elements: 1
Valency of oxygen: 2
Oxides of group 1 elements:
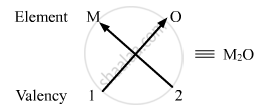
Formula of the oxides of group 1 is M2O, where M is the group 1 element and O is oxygen.
(ii) Valency of group 13 elements: 3
Valency of halogens: 1
Halides of group 13 elements:
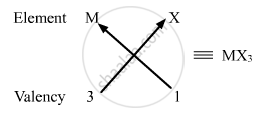
Formula of the halides of group 13 is MX3, where M is the group 13 element and X is halogen.
(iii) Valency of group 2 elements: 2
Valency of group 16 elements: 2
Compounds of group 2 and group 16 elements:
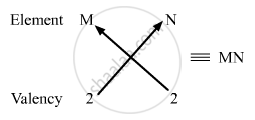
Formula of the compounds of group 2 and 16 is MN, where M is the group 2 element and N is the group 16 element.
APPEARS IN
संबंधित प्रश्न
Given alongside is a part of the periodic table. As we move vertically downward from Li to Fr:
| Li | Be |
| Na | |
| K | |
| Rb | |
| Cs | |
| Fr | Ra |
What happens to their metallic character?
name the elements in period 1.
Arrange the following as per instruction given in the bracket.
K, Pb, Ca, Zn (increasing reactivity)
Atomic numbers of elements A, B, C, D, E, F are 8, 7, 11, 12, 13 and 9 respectively. State the type of ions they form.
An element belongs to the third period and Group IIIA (13) of the periodic table. State: the name of the element.
Moving down in the second group, number of valence electrons ______.
The position of certain elements in the Modern Periodic Table is shown below:

Using the above table answer the following questions giving reasons in each case :
(i) Which element will form only covalent compounds?
(ii) Which element is a non-metal with valency 2?
(iii) Which element is a metal with valency 2?
(iv) Out of H, C, and F which has the largest atomic size?
(v) To which family does H, C, and F belong?
Name or state following with reference to the element of the first three periods of the periodic table.
The valency of elements in group 1 [I–A].
Name or state following with reference to the element of the first three periods of the periodic table.
The non-metallic element present in period 3 other than sulphur and chlorine.
Name or state following with reference to the element of the first three periods of the periodic table.
A non-metal in period 2 which is tetravalent.
