Advertisements
Advertisements
प्रश्न
Complete and balance the following chemical equations:
`NaHCO_3` 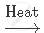
उत्तर
`2NaHCO_3` `Na_2 CO_3 + CO_2 + H_2O`
`Na_2 CO_3 + CO_2 + H_2O`
APPEARS IN
संबंधित प्रश्न
A student takes about 2 mL ethanoic acid in a dry test tube and adds a pinch of sodium hydrogen carbonate to it. He reports the following observations:
I. Immediately a colourless and odourless gas evolves with a brisk effervescence.
II. The gas turns lime water milky when passed through it.
III. The gas burns with an explosion when a burning splinter is brought near it.
IV. The gas extinguishes the burning splinter that is brought near it.
The correct observations are
(A) I, II and III
(B) II, III and IV
(C) III, IV and I
(D) I, II and IV
The discomfort caused by indigestion due to overeating can be cured by taking:
Name an acid which is present in baking powder.
Which compound is used as an antacid in medicine : NaHCO3 or Na2CO3?
Name two constituents of baking powder.
How does baking powder differ from baking soda?
Consider the following substances:
NaCl, Ca(OH)2, NaHCO3, NH3, Na2CO3, H2O, Cl2, CO2, CaSO4.2H2O, 2CaSO4.H2O, CaOCl2
Which compound is a part of baking powder?
Write the chemical formula.
Baking soda
Salt 'P', commonly used in bakery products, on heating gets converted into another salt 'Q' which itself is used for the removal of hardness of water and a gas 'R' is evolved. The gas 'R', when passed through freshly prepared lime water, turns milky. Identify 'P', 'Q' and 'R', giving a chemical equation for the justification of your answer.
NaHCO, formed by reaction of ____________.
