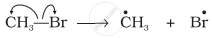Advertisements
Advertisements
प्रश्न
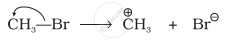
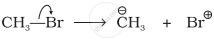
Covalent bond can undergo fission in two different ways. The correct representation involving a heterolytic fission of CH3 – Br is:
विकल्प
उत्तर
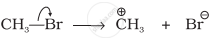
Explanation:
Br is more electronegative than C, hence heterolytic fission takes place. Electrons displace from carbon to Br. Therefore, CH3 gets positive charge and Br gets negative charge.
APPEARS IN
संबंधित प्रश्न
For the following bond cleavages, use curved-arrows to show the electron flow and classify as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion.
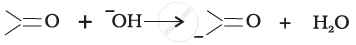
For the following bond cleavages, use curved-arrows to show the electron flow and classify as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion.
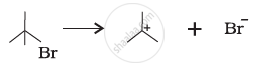
For the following bond cleavages, use curved-arrows to show the electron flow and classify as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion.
Which of the following carbocation is most stable?
What is the correct order of decreasing stability of the following cations.
| I. | \[\ce{CH3 - \overset{⊕}{C}H - CH3}\] |
| II. | \[\ce{CH3 - \overset{⊕}{C}H - OCH3}\] |
| III. | \[\ce{CH3 - \overset{⊕}{C}H - CH2 - OCH3}\] |
Write structures of various carbocations that can be obtained from 2-methylbutane. Arrange these carbocations in order of increasing stability.
Which of the following carbocation's is most stable?
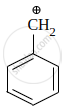 |
\[\ce{CH2 = \overset{⊕}{C}H}\] | \[\ce{CH3 - \overset{⊕}{C}H2}\] | \[\ce{HC ≡ \overset{⊕}{C}}\] |
| A | B | C | D |
The correct order of stability of given carbocation is:
A solution of (–) – 1 – chloro–1–phenylethane in toluene racemises slowly in the presence of a small amount of SbCl5, due to the formation of ______.
The increasing order of stability of the following free radicals is ______.