Advertisements
Advertisements
प्रश्न
Draw diagram for bonding in ethene with sp2 Hybridisation.
उत्तर


C2H4
APPEARS IN
संबंधित प्रश्न
Explain the formation of H2 molecule on the basis of valence bond theory.
Identify the type of overlap present in F2. Explain diagrammatically.
Identify the type of overlap present in H-F molecule. Explain diagrammatically.
Complete the following Table.
| Molecule | Type of Hybridization | Type of bonds | Geometry | Bond angle |
| CH4 | - | 4C-H 4σ bonds |
Tetrahedral | - |
| NH3 | sp3 | 3N-H 3σ bonds 1 lone pair |
- | - |
| H2O | - | - | angular | 104.5° |
| BF3 | sp2 | - | - | 120° |
| C2H4 | - | - | - | 120° |
| BeF2 | - | 2 Be-F | Linear | - |
| C2H2 | sp | (3σ+2π) 1C-C σ 2C-H σ 2C-C π |
- | - |
Mention the steps involved in Hybridization.
The ratio of number of sigma (σ) and pi (л) bonds in 2- butynal is ______.
Which one of the following is the likely bond angles of sulphur tetrafluoride molecule?
According to Valence bond theory, a bond between two atoms is formed when ______.
In ClF3, NF3 and BF3 molecules the chlorine, nitrogen and boron atoms are ______.
Which of these represents the correct order of their increasing bond order.
What is a pi - bond?
Considering x-axis as the molecular axis which out of the following will form a sigma bond.
1s and 2py
Considering x-axis as the molecular axis which out of the following will form a sigma bond.
2px and 2py
Considering x-axis as the molecular axis which out of the following will form a sigma bond.
2px and 2pz
Considering x-axis as the molecular axis which out of the following will form a sigma bond.
1s and 2pz
Which of the following molecule contain 50% p-character of hybrid orbital in C atom?
The number of sigma bonds in paracetamol is ____________.
The overlap of orbitals involved in the formation of C - Br bond in vinyl bromide is ______.
If the electronic configuration of an element is 1s2 2s2 2p6 3s2 3p6 3d2 4s2, the four electrons involved in chemical bond formation will be ______.
Why does type of overlap given in the following figure not result in bond formation?
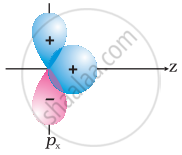 |
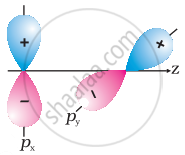 |
Briefly describe the valence bond theory of covalent bond formation by taking an example of hydrogen. How can you interpret energy changes taking place in the formation of dihydrogen?
Match List - I with List - II.
| List - I | List - II | ||
| (a) | \[\ce{PCl5}\] | (i) | Square pyramidal |
| (b) | \[\ce{SF6}\] | (ii) | Trigonal planar |
| (c) | \[\ce{BrF5}\] | (iii) | Octahedral |
| (d) | \[\ce{BF3}\] | (iv) | Trigonal bipyramidal |
Choose the correct answer from the options given below.
