Advertisements
Advertisements
Question
Draw diagram for bonding in ethene with sp2 Hybridisation.
Solution


C2H4
APPEARS IN
RELATED QUESTIONS
Draw an orbital diagram of Fluorine molecule
Distinguish between sigma and pi bond.
Display electron distribution around the oxygen atom in the water molecule and state the shape of the molecule, also write the H-O-H bond angle.
Give reasons for need of Hybridization
Explain geometry of methane molecule on the basis of Hybridization.
Give a reason for the sigma (σ) bond is stronger than the Pi (π) bond.
Give a reason for carbon is tetravalent in nature.
Identify the type of overlap present in F2. Explain diagrammatically.
Complete the following Table.
| Molecule | Type of Hybridization | Type of bonds | Geometry | Bond angle |
| CH4 | - | 4C-H 4σ bonds |
Tetrahedral | - |
| NH3 | sp3 | 3N-H 3σ bonds 1 lone pair |
- | - |
| H2O | - | - | angular | 104.5° |
| BF3 | sp2 | - | - | 120° |
| C2H4 | - | - | - | 120° |
| BeF2 | - | 2 Be-F | Linear | - |
| C2H2 | sp | (3σ+2π) 1C-C σ 2C-H σ 2C-C π |
- | - |
Mention the steps involved in Hybridization.
The ratio of number of sigma (σ) and pi (л) bonds in 2- butynal is ______.
Which one of the following is the likely bond angles of sulphur tetrafluoride molecule?
According to Valence bond theory, a bond between two atoms is formed when ______.
In ClF3, NF3 and BF3 molecules the chlorine, nitrogen and boron atoms are ______.
XeF2 is isostructural with ______.
Define σ – bond.
Considering x-axis as the molecular axis which out of the following will form a sigma bond.
1s and 2py
Considering x-axis as the molecular axis which out of the following will form a sigma bond.
2px and 2py
Considering x-axis as the molecular axis which out of the following will form a sigma bond.
2px and 2pz
Considering x-axis as the molecular axis which out of the following will form a sigma bond.
1s and 2pz
Ethene molecule has ____________ sp2 -s σ bond(s), ____________ sp2 -sp2 σ bond(s) and ____________ p-p π bond(s).
Which of the following molecule contain 50% p-character of hybrid orbital in C atom?
The number of sigma bonds in vanillin is ____________.
If the electronic configuration of an element is 1s2 2s2 2p6 3s2 3p6 3d2 4s2, the four electrons involved in chemical bond formation will be ______.
Why does type of overlap given in the following figure not result in bond formation?
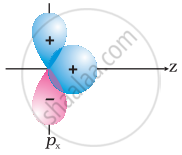 |
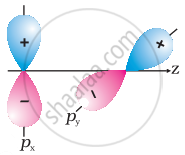 |
Which of the following statements is INCORRECT according to the valence bond theory?
