Advertisements
Advertisements
Question
Display electron distribution around the oxygen atom in the water molecule and state the shape of the molecule, also write the H-O-H bond angle.
Solution
Electron distribution around oxygen atom in water molecule:

Shape of water molecule: Angular or V-shaped
H–O–H bond angle = 104°35’
APPEARS IN
RELATED QUESTIONS
Draw diagram for bonding in ethene with sp2 Hybridisation.
Draw an orbital diagram of Fluorine molecule
Distinguish between sigma and pi bond.
Give reasons for need of Hybridization
Explain geometry of methane molecule on the basis of Hybridization.
Give a reason for carbon is tetravalent in nature.
Identify the type of overlap present in H2. Explain diagrammatically.
Give the type of overlap by which the pi (π) bond is formed.
Mention the steps involved in Hybridization.
The ratio of number of sigma (σ) and pi (л) bonds in 2- butynal is ______.
Which one of the following is the likely bond angles of sulphur tetrafluoride molecule?
In ClF3, NF3 and BF3 molecules the chlorine, nitrogen and boron atoms are ______.
When ones and three p orbitals hybridise,
The correct order of O – O bond length in hydrogen peroxide, ozone and oxygen is
What is a pi - bond?
Considering x-axis as the molecular axis which out of the following will form a sigma bond.
1s and 2py
Ethene molecule has ____________ sp2 -s σ bond(s), ____________ sp2 -sp2 σ bond(s) and ____________ p-p π bond(s).
Which of the following molecule contain 50% p-character of hybrid orbital in C atom?
The number of sigma bonds in paracetamol is ____________.
The overlap of orbitals involved in the formation of C - Br bond in vinyl bromide is ______.
Why does type of overlap given in the following figure not result in bond formation?
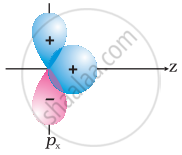 |
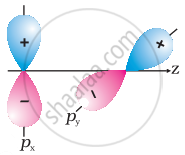 |
Which of the following statements is INCORRECT according to the valence bond theory?
Match List - I with List - II.
| List - I | List - II | ||
| (a) | \[\ce{PCl5}\] | (i) | Square pyramidal |
| (b) | \[\ce{SF6}\] | (ii) | Trigonal planar |
| (c) | \[\ce{BrF5}\] | (iii) | Octahedral |
| (d) | \[\ce{BF3}\] | (iv) | Trigonal bipyramidal |
Choose the correct answer from the options given below.
