Advertisements
Advertisements
प्रश्न
Draw the structure of the following atoms showing the nucleus containing – protons, neutrons and the orbits with the respective electron:
Lithium [At. no. = 3, Mass no. = 7]
उत्तर
Structure of atoms:
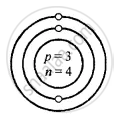
Z is Atomic Number A is the mass number
Lithium \[\ce{^7_3Li}\]
Z = 3 = p = e
K L
e = 3 = 2, 1
A = p + n
7 = 3 + 3
∴ n = 7 − 3 = 4
APPEARS IN
संबंधित प्रश्न
TRUE \ FALSE
After the emission of a beta-particle, the atomic number of the atom increases by one.
Fill in the blanks- The number of protons in the nucleus of an atom is called its ....................
From the symbol `""_15^31"P"`, state :
(i) mass number of phosphorus,
(ii) atomic number of phosphorus, and
(iii) electron configuration of phosphorus.
Draw the orbital diagram of `""_20^40"Ca"^(2+)` ion and state the number of three fundamental particles present in it.
Draw the atomic diagram of the following element showing the distribution of – protons, neutrons and the electrons in the various shell of the atom.
Argon – \[\ce{^40_18Ar}\]
[The upper number represent the – mass number and the lower number represent the – atomic number e.g. calcium – mass number = 40, atomic number = 20]
Draw the atomic diagram of the following element showing the distribution of – protons, neutrons and the electrons in the various shell of the atom.
Calcium – \[\ce{^40_20Ca}\]
[The upper number represent the – mass number and the lower number represent the – atomic number e.g. calcium – mass number = 40, atomic number = 20]
‘A’ is denoted by ______.
Mass No. 27 is for the element ______.
Match the following:
| Column A | Column B | ||
| 1. | K shell | a. | 8 |
| 2. | L shell | b. | 32 |
| 3. | M shell | c. | 2 |
| 4. | N shell | d. | 18 |
A group of elements in the periodic table is given below (Boron is the first member of the group and Thallium is the last).
Boron, Aluminium, Gallium, Indium, Thallium
Answer the following question in relation to the above group of elements:
The atomic number of boron is 5. Write the chemical formula of the compound formed when boron reacts with chlorine.
