Advertisements
Advertisements
प्रश्न
Draw the structure of the following atoms showing the nucleus containing – protons, neutrons and the orbits with the respective electron:
Lithium [At. no. = 3, Mass no. = 7]
उत्तर
Structure of atoms:
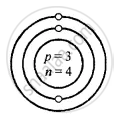
Z is Atomic Number A is the mass number
Lithium \[\ce{^7_3Li}\]
Z = 3 = p = e
K L
e = 3 = 2, 1
A = p + n
7 = 3 + 3
∴ n = 7 − 3 = 4
APPEARS IN
संबंधित प्रश्न
Explain the Atomic number with example.
Complete the following table.
| Atomic number | Mass number | Number of Neutrons | Number of protons | Number of electrons | Name of the Atomic species |
| 9 | - | 10 | - | - | - |
| 16 | 32 | - | - | - | Sulphur |
| - | 24 | - | 12 | - | - |
| - | 2 | - | 1 | - | - |
| - | 1 | 0 | 1 | 0 | - |
Fill in the blanks- The number of protons in the nucleus of an atom is called its ....................
Fill in the blank of the following statement :
The total number of protons and neutrons in the nucleus of an atom is called its _________.
From the symbol `""_15^31"P"`, state :
(i) mass number of phosphorus,
(ii) atomic number of phosphorus, and
(iii) electron configuration of phosphorus.
What is meant by the 'atomic number of an element'?
If an atom ‘A’ has atomic number 19 and mass number 39, state –
- Its electronic configuration.
- The number of valence electrons it possesses.
The diagram represents an isotope of hydrogen [H]. Answer the following:

Atomic no. = 1
Mass no. = 1
Do isotopes have the same atomic number or the same mass number?
The diagram represents an isotope of hydrogen [H]. Answer the following:

Atomic no. = 1
Mass no. = 1
If an isotope of ‘H’ has mass no. = 2, how many electrons does it have.
An element ‘A’ has mass number 23 and atomic number 11. State the –
- no. of neutrons in its shell,
- electronic configuration of the element ‘A’.
