Advertisements
Advertisements
Question
Draw the structure of the following atoms showing the nucleus containing – protons, neutrons and the orbits with the respective electron:
Lithium [At. no. = 3, Mass no. = 7]
Solution
Structure of atoms:
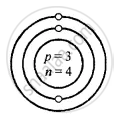
Z is Atomic Number A is the mass number
Lithium \[\ce{^7_3Li}\]
Z = 3 = p = e
K L
e = 3 = 2, 1
A = p + n
7 = 3 + 3
∴ n = 7 − 3 = 4
APPEARS IN
RELATED QUESTIONS
Explain the Atomic number with example.
Write true or false for the following statement
An atom on the whole has a positive charge.
Multiple Choice Questions
The sum of number of protons and number of neutrons present in the nucleus of an atom is called its
Name or state the following:
Elements having same mass number but different atomic number
The diagram represents an isotope of hydrogen [H]. Answer the following:

Atomic no. = 1
Mass no. = 1
If an isotope of ‘H’ has mass no. = 3, how many neutrons does it have.
Draw the structure of the following atoms showing the nucleus containing – protons, neutrons and the orbits with the respective electron:
Carbon [At. no. = 6, Mass no. = 12]
Elements A, B and C have atomic numbers 9, 20 and 10 respectively.
- State which one is
(1) a non-metal,
(2) a metal,
(3) chemically inert.[A,B,C] - Write down the formula of the compound formed by two of the above elements.[BA2]
Show diagrammatically the electron distributions in a sodium atom and a sodium ion and also give their atomic number.
Give short answer:
what is the atomic mass number? mention its formula.
What is the relation between mass number & atomic number?
