Advertisements
Advertisements
Question
Draw the structure of the following atoms showing the nucleus containing – protons, neutrons and the orbits with the respective electron:
Carbon [At. no. = 6, Mass no. = 12]
Solution
Structure of atoms:
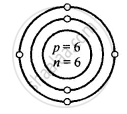
Z is Atomic Number and A is the mass number
Carbon \[\ce{^12_6C}\]
Z = 6 = p = e
K L
e = 6 = 2, 4
A = p + n
12 = 6 + n
∴ n = 12 − 6 = 6
APPEARS IN
RELATED QUESTIONS
Explain the Atomic number with example.
The composition of the nuclei of two atomic species X and Y are given as under
| X | Y | |
| Protons | 6 | 6 |
| Neutrons | 6 | 8 |
Give the mass numbers of X and Y. What is the relation between the two species?
Multiple Choice Questions
If the atomic number of an atom is 17 and mass number is 35 then number of neutron will be
Tick the most appropriate answer.
Which of these have similar chemical properties but different set of physical properties and mass number?
- isobars
- elements
- isotopes
- none of these
TRUE \ FALSE
After the emission of a beta-particle, the atomic number of the atom increases by one.
Name the particles which actually determine the mass of an atom.
The mass number of an element is 23 and it contains 11 electrons. What is the number of protons and neutrons in it? What is the atomic number of the element?
Explain the following terms.
- Proton
- Electron
- Neutron.
What is the relation between mass number & atomic number?
Element ‘P' has electronic configuration 2,8,8,1. The number of chlorine atoms present in the chloride of ‘P’ is ______.
