Advertisements
Advertisements
Question
Elements A, B and C have atomic numbers 9, 20 and 10 respectively.
- State which one is
(1) a non-metal,
(2) a metal,
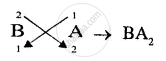
(3) chemically inert.[A,B,C] - Write down the formula of the compound formed by two of the above elements.[BA2]
Solution
- 9A 20B 10C
Atomic number of:
A = 9 = number of electrons = 2, 7
B = 20 = number of electons = 2, 8, 8, 2
C = 10 = number of electrons = 2, 8
(1) A non-metal = A
(2) A metal = B
(3) Chemically inert = C - Compound formed is A1−, B2+
APPEARS IN
RELATED QUESTIONS
Complete the following table.
| Atomic number | Mass number | Number of Neutrons | Number of protons | Number of electrons | Name of the Atomic species |
| 9 | - | 10 | - | - | - |
| 16 | 32 | - | - | - | Sulphur |
| - | 24 | - | 12 | - | - |
| - | 2 | - | 1 | - | - |
| - | 1 | 0 | 1 | 0 | - |
What are the atomic numbers of elements whose outermost electrons is represented by 2p3.
Dalton said that ______ could not be divided.
Fill in the blanks.
Isotopes are the atoms of ______ element having the ______ atomic number but ______ mass number.
Tick the most appropriate answer.
Which of these have similar chemical properties but different set of physical properties and mass number?
- isobars
- elements
- isotopes
- none of these
An atom of an element has two electrons in the M shell.
What is the (a) atomic number (b) number of protons in this element?
The diagram represents an isotope of hydrogen [H]. Answer the following:

Atomic no. = 1
Mass no. = 1
If an isotope of ‘H’ has mass no. = 3, how many neutrons does it have.
The atomic number of an element is 9, it has 10 neutrons. Find the element from the periodic table. What will be its mass number?
The atomic number and the mass number of an element is 26 and 56 respectively. Calculate the number of electrons, protons and neutrons in its atom. Draw the structure.
Compare the charge and mass of protons and electrons.
