Advertisements
Online Mock Tests
Chapters
![Viraf J. Dalal solutions for Simplified ICSE Chemistry [English] Class 9 chapter 4 - Atomic Structure & Chemical Bonding Viraf J. Dalal solutions for Simplified ICSE Chemistry [English] Class 9 chapter 4 - Atomic Structure & Chemical Bonding - Shaalaa.com](/images/simplified-icse-chemistry-english-class-9_6:15cbe1f7c39e424a9ab3e108260ad612.jpg)
Advertisements
Solutions for Chapter 4: Atomic Structure & Chemical Bonding
Below listed, you can find solutions for Chapter 4 of CISCE Viraf J. Dalal for Simplified ICSE Chemistry [English] Class 9.
Viraf J. Dalal solutions for Simplified ICSE Chemistry [English] Class 9 4 Atomic Structure & Chemical Bonding Exercise
From the symbol \[\ce{^4_2He}\] for the element helium, write down the mass number and the atomic number of the element.
Sulphur has an atomic number of 16 and a mass number of 32. State the number of protons and neutrons present in the nucleus of sulphur.
\[\ce{^24_12Mg}\] and \[\ce{^26_12Mg}\] are symbols of isotopes of magnesium.
(a) Compare the atoms of these isotopes with respect to :
i. the composition of their nuclei
ii. their electronic configurations
(b) Give reasons why the two isotopes of magnesium have different mass numbers.
Five atoms are labelled V to Z
| Atoms | Mass Number | Atomic Number |
| V | 40 | 20 |
| W | 19 | 9 |
| X | 7 | 3 |
| Y | 16 | 8 |
| Z | 14 | 7 |
- Which one of these atoms
(1) contains 7 protons;
(2) has an electronic configuration 2, 7? - Write down the formula of the compound formed by atoms X and Y.
Elements X, Y, Z have atomic numbers 6, 9 and 12 respectively. Which one:
- forms anion – negative ion;
- forms cation – positive ion;
- has 4 electrons in the outermost orbit. [Y, Z, X]
Write down the electronic configuration of the following
- 2713X,
- 3517Y.
Write down the number of electrons in X and neutrons in Y and the formula of the compound formed by X and Y.[XY3]
According to Dalton’s Atomic Theory, atoms of the same element are identical in all respects. But according to the Modern Atomic Theory, this postulate is proved wrong. Explain.
What are isotopes?
The atom of aluminium is represented by 27Al13. Write down the number of electrons in the different orbits or shells in one atom of aluminium.
Define: Proton
Define the term of atomic number.
Define the term of mass number.
The atom of aluminium is represented by 27Al13. Write down the number of protons in the different orbits or shells in one atom of aluminium.
What is the significance of the number of protons found in the atoms of different elements?
Define the term of the electron.
The atom of aluminium is represented by 27Al13. Write down the number of neutrons in the different orbits or shells in one atom of aluminium.
The atom of aluminium is represented by 27Al13. Write down the number of the arrangement of electrons in the different orbits or shells in one atom of aluminium.
Give a simple diagram to show the arrangement of the electrons in an atom of sulphur.
Chlorine is an element of atomic number 17. It is a mixture of two isotopes having mass number of 35 and 37.
- What is meant by “atomic number of an element”? What do you understand by an ‘atom’
- Write down the electronic configuration of the chlorine atom.
- State the number of protons, electrons, and neutrons in the following isotopes: 35Cl17, 37Cl17
- Explain why the two atoms in (iii) above have the same chemical reactions.
- If molten magnesium chloride is electrolyzed suggest a suitable electrode [anode].
Ordinary chlorine gas has two isotopes: \[\ce{^35_17Cl}\] and \[\ce{^37_17Cl}\] in the ratio of 3 : 1. Calculate the relative atomic mass [atomic weight] of chlorine.
Name the element which does not contain any neutrons in its nucleus.
What is the relation between the number of protons and the number of electrons in an atom?
Write down the mass number of the atom having 20 neutrons and 15 protons.
Write down the number of neutrons in the nucleus of an atom having atomic number 17 and mass number 37.
Elements A, B and C have atomic numbers 9, 20 and 10 respectively.
- State which one is
(1) a non-metal,
(2) a metal,
(3) chemically inert.[A,B,C] - Write down the formula of the compound formed by two of the above elements.[BA2]
What would be the reason for an element to have atoms with different mass numbers?
Copy and complete the following table relating to the atomic structure of some elements:
| Element | Atomic Number | Mass Number | Number of protons | Number of Neutrons | Number of Electrons |
| Beryllium | 4 | 9 | |||
| Fluorine | 9 | 10 | |||
| Sodium | 12 | 11 | |||
| Aluminium | 27 | 13 | |||
| Phosphorus | 31 | 15 |
Define: Proton
Define: Electron
Define: Neutron
The electronic structure [configuration] of fluorine can be written as 2, 7. In a similar way give the electronic configuration of aluminium
The electronic structure [configuration] of fluorine can be written as 2, 7. In a similar way give the electronic configuration of phosphorus
Viraf J. Dalal solutions for Simplified ICSE Chemistry [English] Class 9 4 Atomic Structure & Chemical Bonding Additional Questions
State Dalton's atomic theory.
How does the Modern atomic theory contradict and correlate with Dalton's atomic theory?
Explain in brief the experimental proof which led to the discovery of –
Electrons
Explain in brief the experimental proof which led to the discovery of –
Protons
Explain in brief the experimental proof which led to the discovery of –
Atomic nucleus
Explain in brief the experimental proof which led to the discovery of –
Neutrons
State in brief the drawbacks of Rutherford's atomic model correlating them with the postulates of Bohr’s atomic model.
What is meant by the term subatomic particles?
What is meant by the term nucleus
What is meant by the term orbits
Define the term of atomic number.
Define the term of mass number.
Represent a proton ‘p’ in term of its symbol showing the subscript and superscript value.
Represent an electron ‘e’ in term of its symbol showing the subscript and superscript value.
Represent a neutron ‘n’ in term of its symbol showing the subscript and superscript value.
What are ‘energy levels’?
Explain the arrangement and distribution of electrons in the various shells with reference to an atom in general and to an atom of potassium ‘3919K’ with special reference to the 2n2 rule.
An element ‘A’ has mass number 23 and atomic number 11. State the –
- no. of neutrons in its shell,
- electronic configuration of the element ‘A’.
The following element is given \[\ce{_3U}\]. State the electronic configuration and state whether it is a metal, non-metal, or inert gas.
The following element is given \[\ce{_6V}\]. State the electronic configuration and state whether it is a metal, non-metal, or inert gas.
The following element is given \[\ce{_9W}\]. State the electronic configuration and state whether it is a metal, non-metal, or inert gas.
The following element is given \[\ce{_14X}\]. State the electronic configuration and state whether it is a metal, non-metal, or inert gas.
The following element is given \[\ce{_18Y}\]. State the electronic configuration and state whether it is a metal, non-metal, or inert gas.
The following element is given \[\ce{_20Z}\]. State the electronic configuration and state whether it is a metal, non-metal, or inert gas.
Draw the geometric atomic structure of the following atom showing the number of electrons, protons and neutrons in them:
\[\ce{^12_6C}\]
Draw the geometric atomic structure of the following atom showing the number of electrons, protons and neutrons in them:
\[\ce{^23_11Na}\]
Draw the geometric atomic structure of the following atom showing the number of electrons, protons and neutrons in them:
\[\ce{^31_15P}\]
Draw the geometric atomic structure of the following atom showing the number of electrons, protons and neutrons in them:
\[\ce{^39_19K}\]
Draw the geometric atomic structure of the following atom showing the number of electrons, protons and neutrons in them:
\[\ce{^40_20Ca}\]
What are isotopes?
Give the reason why isotopes have the same chemical but different physical properties.
Draw the geometric atomic structure of the three isotopes of hydrogen and the two isotopes of chlorine.
Four elements A, B, C, D are given :
- A shows the presence of 20 neutrons, 17 protons and 17 electrons.
- B shows the presence of 18 neutrons, 17 protons and 17 electrons.
- C shows the presence of 10 neutrons, 9 protons and 10 electrons.
- D shows the presence of 4 neutrons, 3 protons and 2 electrons.
State which of the above is –
- an anion
- a cation
- a pair of isotopes.
Write the formula of the compound formed between D and C.
What are the noble gases?
Give a reason why noble gases have a stable electronic configuration.
Explain the reason for the chemical activity of an atom with reference to its electronic configuration.
Differentiate between the term Stable and unstable electronic configuration.
Differentiate between the term Duplet and octet rule.
Explain the octet rule for the formation of –
Sodium chloride from a sodium atom and a chlorine atom.
Explain the octet rule for the formation of –
Nitrogen molecule from two nitrogen atoms.
Viraf J. Dalal solutions for Simplified ICSE Chemistry [English] Class 9 4 Atomic Structure & Chemical Bonding Atomic Structure
Select the correct answer from the answer in the bracket to complete the sentence.
An element has electronic configuration 2, 8, 1 and 12 neutrons. Its mass no. is ________.
11
23
12
Select the correct answer from the answer in the bracket to complete the sentence.
The maximum number of electrons in M-shell is _______.
8
32
18
Select the correct answer from the answer in the bracket to complete the sentence.
Isotopes have same _________.
no. of neutrons
electronic configuration
atomic masses
Select the correct answer from the answer in the bracket to complete the sentence.
An ________ is capable of independent existence in solution.
atom
ion
Select the correct answer from the answer in the bracket to complete the sentence.
An atom with electronic configuration 2, 7 and mass number 19 will have ________ neutrons.
8
10
12
Give a reason
Physical properties of isotopes are different.
Give a reason for the following:
The mass number of an atom is slightly less than the actual atomic mass.
Give a reason for the following:
The shells surrounding the nucleus of an atom are also called ‘energy levels’.
Give a reason for the following:
Helium is chemical extremely unreactive.
Give a reason for the following:
The mass number of an atom is slightly less than the actual atomic mass.
Differentiate between the following term:
Electron and proton
Differentiate between the following term:
Atomic number and mass number
Differentiate between the following term:
Nucleus and nucleons
Differentiate between the following term:
Valence shell and penultimate shell
Differentiate between the term Duplet and octet rule.
Name or state the following.
The three isotopes of hydrogen.
Name or state the following.
Two elements having the same number of protons and electrons but a different number of neutrons.
Name or state the following.
The valency of an element whose electronic configuration is 2, 8, 3.
Name the following:
The shell closest to the nucleus of an atom
Name the following:
An element having valency 'zero'
State the number of neutrons in each of the atoms A to E. Also state which of the atoms A to E is a metal.
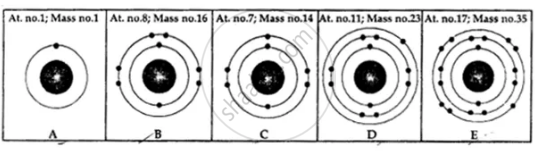
Match the elements A to E in List 1 with their valencies in List 2 and with their nature in List 3.
| List 1 [Elements] | List 2 [Valency] | List 3 |
| A: At. no. 7, Mass no. 14 | 1. −3 | X: Metal |
| B: Elec. conf. 2, 8 | 2. +1 | Y: Non-metal |
| C: Neutrons 14, electrons 13 | 3. +3 | Z: Noble gas |
| D: Neutrons 22, protons 18 | 4. +2 | |
| E: Elec. config. 2, 8, 1 | 5. 0 |
Solutions for 4: Atomic Structure & Chemical Bonding
![Viraf J. Dalal solutions for Simplified ICSE Chemistry [English] Class 9 chapter 4 - Atomic Structure & Chemical Bonding Viraf J. Dalal solutions for Simplified ICSE Chemistry [English] Class 9 chapter 4 - Atomic Structure & Chemical Bonding - Shaalaa.com](/images/simplified-icse-chemistry-english-class-9_6:15cbe1f7c39e424a9ab3e108260ad612.jpg)
Viraf J. Dalal solutions for Simplified ICSE Chemistry [English] Class 9 chapter 4 - Atomic Structure & Chemical Bonding
Shaalaa.com has the CISCE Mathematics Simplified ICSE Chemistry [English] Class 9 CISCE solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. Viraf J. Dalal solutions for Mathematics Simplified ICSE Chemistry [English] Class 9 CISCE 4 (Atomic Structure & Chemical Bonding) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. Viraf J. Dalal textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Simplified ICSE Chemistry [English] Class 9 chapter 4 Atomic Structure & Chemical Bonding are Chemical Bond, History of Atom, Elements, Atoms: Building Blocks of Matter, Discovery of Charged Particles in Matter, Electrons (e), Protons (p), Nucleus, Neutrons (n), J. J. Thomson’s Atomic Model, Lord Rutherford’s Atomic model, Neils Bohr’s Model of an Atom, Structure of an Atom, Atomic Number (Z), Mass Number (A), and Number of Neutrons (n), Atomic Mass, Electronic Configuration of Atom, Reason for Chemical Activity of an Atom, Isotopes, Isobars, Types of Covalent Bond, Formation of Covalent Bond, The Covalent Bond, Ionic or Electrovalent Bond, Ionic or Electrovalent Bond, Valency, Dalton’s Atomic Theory.
Using Viraf J. Dalal Simplified ICSE Chemistry [English] Class 9 solutions Atomic Structure & Chemical Bonding exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in Viraf J. Dalal Solutions are essential questions that can be asked in the final exam. Maximum CISCE Simplified ICSE Chemistry [English] Class 9 students prefer Viraf J. Dalal Textbook Solutions to score more in exams.
Get the free view of Chapter 4, Atomic Structure & Chemical Bonding Simplified ICSE Chemistry [English] Class 9 additional questions for Mathematics Simplified ICSE Chemistry [English] Class 9 CISCE, and you can use Shaalaa.com to keep it handy for your exam preparation.
