Advertisements
Advertisements
Question
Draw the geometric atomic structure of the following atom showing the number of electrons, protons and neutrons in them:
\[\ce{^40_20Ca}\]
Solution
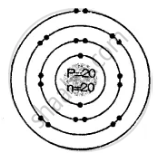
Geometric atomic structure of:
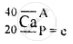
A = P + n
40 = 20 + n
∴ n = 40 − 20 = 20 neutrons
e = 20
Electronic arrangement 2, 8, 8, 2
K L M N
APPEARS IN
RELATED QUESTIONS
Five atoms are labelled from A to E.
| Atoms | Mass No. | Atomic No. |
| A | 40 | 20 |
| B | 19 | 9 |
| C | 7 | 3 |
| D | 16 | 8 |
| E | 14 | 7 |
(a) Which one of these atoms:
(i) contains 7 protons,
(ii) has an electronic configuration 2, 7?
(b) Write down the formula of the compound formed between C and D.
(c) predict which are: (i) metals, (ii) non-metals?
The following table shows the electronic configuration of the elements W, X, Y, Z:
|
Element |
W |
X |
Y |
Z |
|
Electronic |
2, 8, 1 |
2, 8, 7 |
2, 5 |
1 |
Answer the following question based on the table above:
What is the formula of the compound formed between W and X.
What is an α (alpha) particle?
How are X-rays produced?
Name the following:
An element having valency 'zero'
Compare :
Sodium atom and sodium ion
Give the orbital diagram of the following:
Magnesium chloride
Identify the following reaction as either oxidation or reduction:
O + 2e- → O2-
Identify the following reaction as either oxidation or reduction:
K - e- → K+
How many electrons are required or released by each atom in chlorine to attain the nearest noble gas configuration?
