Advertisements
Advertisements
Question
The following table shows the electronic configuration of the elements W, X, Y, Z:
|
Element |
W |
X |
Y |
Z |
|
Electronic |
2, 8, 1 |
2, 8, 7 |
2, 5 |
1 |
Answer the following question based on the table above:
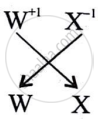
What is the formula of the compound formed between W and X.
Solution
WX is the formula of the compound formed between W and X.
APPEARS IN
RELATED QUESTIONS
Define a chemical bond.
What type of bond is formed between two atoms, when the electronegative difference between them is High ?
How many atoms of each kind are present in the following molecules: calcium oxide, chlorine, water, carbon tetrachloride?
How many electrons are required by each atom mentioned in (a) to attain the nearest noble gas configuration?
Why do they exist as monoatoms in molecules?
Explain fractional atomic mass. What is the fractional mass of chlorine?
Choose the correct option
Which of the following is the correct electronic configuration of potassium?
Potassium (at No.19) and chlorine (at No.17) react to form a compound. Explain the formation of the compound on the basis of reduction.
Write down the electronic configuration of
(i) chlorine atom (ii) chlorine ion
Draw the geometric atomic structure of the following atom showing the number of electrons, protons and neutrons in them:
\[\ce{^12_6C}\]
______ theory explains the formation of molecules.
