Advertisements
Advertisements
प्रश्न
The following table shows the electronic configuration of the elements W, X, Y, Z:
|
Element |
W |
X |
Y |
Z |
|
Electronic |
2, 8, 1 |
2, 8, 7 |
2, 5 |
1 |
Answer the following question based on the table above:
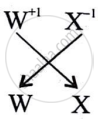
What is the formula of the compound formed between W and X.
उत्तर
WX is the formula of the compound formed between W and X.
APPEARS IN
संबंधित प्रश्न
How many atoms of each kind are present in the following molecules: calcium oxide, chlorine, water, carbon tetrachloride?
How many electrons are required by each atom mentioned in (a) to attain the nearest noble gas configuration?
What is the contribution of the following in atomic structure?
Democritus
Define the term:
orbit
Draw orbit structure diagram of calcium oxide (CaO).
Potassium (at No.19) and chlorine (at No.17) react to form a compound. Explain the formation of the compound on the basis of reducing agent.
\[\ce{^24_12Mg}\] and \[\ce{^26_12Mg}\] are symbols of isotopes of magnesium.
(a) Compare the atoms of these isotopes with respect to :
i. the composition of their nuclei
ii. their electronic configurations
(b) Give reasons why the two isotopes of magnesium have different mass numbers.
Draw the geometric atomic structure of the following atom showing the number of electrons, protons and neutrons in them:
\[\ce{^39_19K}\]
An atom X with atomic number 20 combines with atom Y with atomic number 8. Draw the dot structure for the formation of the molecule XY.
The element that would form cation due to the loss of electrons during the chemical reaction is ______.
How many electrons are required or released by each atom in carbon tetrachloride to attain the nearest noble gas configuration?
