Advertisements
Advertisements
प्रश्न
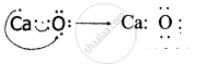
An atom X with atomic number 20 combines with atom Y with atomic number 8. Draw the dot structure for the formation of the molecule XY.
उत्तर
Atom X is Calcium with atomic number 20 and atom Y is Oxygen with atomic number 8.
APPEARS IN
संबंधित प्रश्न
What type of bond is formed between two atoms, when the electronegative difference between them is low ?
What is an α (alpha) particle?
Why do they exist as monoatoms in molecules?
Match the atomic numbers 4, 14, 8, 15 and 19 with each of the following:
- A solid non-metal of valency 3.
- A gas of valency 2.
- A metal of valency 1.
- A non-metal of valency 4.
Identify the following reaction as either oxidation or reduction:
Fe3+ + e- → Fe2+
Five atoms are labelled V to Z
| Atoms | Mass Number | Atomic Number |
| V | 40 | 20 |
| W | 19 | 9 |
| X | 7 | 3 |
| Y | 16 | 8 |
| Z | 14 | 7 |
- Which one of these atoms
(1) contains 7 protons;
(2) has an electronic configuration 2, 7? - Write down the formula of the compound formed by atoms X and Y.
Draw the geometric atomic structure of the following atom showing the number of electrons, protons and neutrons in them:
\[\ce{^12_6C}\]
The element that would form anion by gaining electrons in a chemical reaction is ______.
Choose the odd one out and write the reason:
What is a chemical bond?
