Advertisements
Advertisements
Question
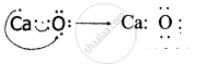
An atom X with atomic number 20 combines with atom Y with atomic number 8. Draw the dot structure for the formation of the molecule XY.
Solution
Atom X is Calcium with atomic number 20 and atom Y is Oxygen with atomic number 8.
APPEARS IN
RELATED QUESTIONS
Match the atomic number 4, 8, 14, 15 and 19 with each of the following:
(a) A solid non-metal of valelncy 3.
(b) A gas of valency 2.
(c) A metal of valency 1.
(d) A non-metal of valency 4
State differences between the properties of ionic compounds and covalent compounds.
What type of bond is formed between two atoms, when the electronegative difference between them is zero?
Give reason
`""_17^35"CI"` and `""_17^37"CI"` do not differ in their chemical reactions.
What do you understand by redox reactions?
\[\ce{^24_12Mg}\] and \[\ce{^26_12Mg}\] are symbols of isotopes of magnesium.
(a) Compare the atoms of these isotopes with respect to :
i. the composition of their nuclei
ii. their electronic configurations
(b) Give reasons why the two isotopes of magnesium have different mass numbers.
Explain the following:
Carbon-12 and carbon-14 both show similar chemical properties.
Draw the electron distribution diagram for the formation of Carbon di oxide (CO2 ) molecule.
Spot the error/correct the wrong statement:
In the formation of compounds, the inner shell electrons of an atom involved in bonding.
Choose the odd one out and write the reason:
