Advertisements
Advertisements
Question
Five atoms are labelled V to Z
| Atoms | Mass Number | Atomic Number |
| V | 40 | 20 |
| W | 19 | 9 |
| X | 7 | 3 |
| Y | 16 | 8 |
| Z | 14 | 7 |
- Which one of these atoms
(1) contains 7 protons;
(2) has an electronic configuration 2, 7? - Write down the formula of the compound formed by atoms X and Y.
Solution
(i) As Z has atomic number of 7, hence it has 7 protons. Electronic configuration of 2, 7 means the atom has 2 + 7 = 9 electrons. Therefore, element W has electronic configuration 2, 7 since its atomic number is 9.
(ii) Number of electron in X is 3. Therefore, electronic configuration = (2, 1). As X will try to lose 1 electron to attain stable state, hence, X has valency [1+]
Number of electron in Y is 8. Therefore, electronic configuration = (2, 6). As Y will try to gain 2 electrons to attain stable state, hence, Y has valency [2−]
Since the valency of X is 1+ and valency of Y is 2−
Formula of the compound:
X1+ Y2−
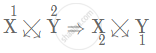
So, we get the formula as X2Y.
APPEARS IN
RELATED QUESTIONS
Elements X, Y, and Z have atomic numbers 6, 9 and 12 respectively. Which one forms an anion?
Give electron dot diagram of the following:
nitrogen
Separate the following compounds into three categories - ionic, polar and covalent compounds; N2, NH4Cl, NH3, NO, NH4NO3, NCl3.
Element X is a metal with a valency 2. Element Y is a non metal with a valency 3.
(a) Write equations to show how X and Y form ions.
(b) If Y is a diatomic gas, write the equation for the direct combination of X and Y to form a compound.
What do you understand by redox reactions?
Identify the following reaction as either oxidation or reduction:
K - e- → K+
Draw the geometric atomic structure of the following atom showing the number of electrons, protons and neutrons in them:
\[\ce{^31_15P}\]
Complete the table give below.
| Element |
Atomic number |
Electron distribution | Valence electrons | Lewis dot structure |
| Lithium | 3 | 1s22s1 | ||
| Boron | 5 | 1s22s22p1 | ||
| Oxygen | 8 | 1s22s22p4 |
Match the following:
| 1. | Atomic bond | a. | Oxygen and hydrogen |
| 2. | Atoms with different electronegativities | b. | acceptor bond |
| 3. | An atom that accepts electron pair | c. | covalent bond |
| 4. | Rusting of iron | d. | donor atom |
| 5. | An atom that provides electron pair | e. | oxidation |
| f. | ionic bond |
What is a chemical bond?
