Advertisements
Advertisements
Question
Complete the table give below.
| Element |
Atomic number |
Electron distribution | Valence electrons | Lewis dot structure |
| Lithium | 3 | 1s22s1 | ||
| Boron | 5 | 1s22s22p1 | ||
| Oxygen | 8 | 1s22s22p4 |
Solution
| Element |
Atomic number |
Electron distribution | Valence electrons | Lewis dot structure |
| Lithium | 3 | 1s22s1 | 1 | 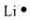 |
| Boron | 5 | 1s22s22p1 | 3 |  |
| Oxygen | 8 | 1s22s22p4 | 6 |  |
APPEARS IN
RELATED QUESTIONS
State differences between the properties of ionic compounds and covalent compounds.
Fill in the blank.
___________ single covalent bonds are formed between the carbon and chlorine atoms.
Elements X, Y, and Z have atomic numbers 6, 9 and 12 respectively. Which one forms a cation?
State the following is a reduction reaction or oxidation reaction.
\[\ce{Fe^2+ - e- -> Fe^3+}\]
Name the following:
Two atoms having the same number of protons and electrons but different number of neutrons.
Give reason
`""_17^35"CI"` and `""_17^37"CI"` do not differ in their chemical reactions.
What do you understand by redox reactions?
Explain the following:
Carbon-12 and carbon-14 both show similar chemical properties.
Complete the following table:
| a. | Dativebond | ______ |
| b. | CaH2 → Ca+H2 | ______ |
| c. | ______ | Reduction |
| d. | ______ | Redox reaction |
The one which is composed of all the three kinds of bond.[ionic, covalent and coordinate bond]
