Advertisements
Advertisements
प्रश्न
Five atoms are labelled V to Z
| Atoms | Mass Number | Atomic Number |
| V | 40 | 20 |
| W | 19 | 9 |
| X | 7 | 3 |
| Y | 16 | 8 |
| Z | 14 | 7 |
- Which one of these atoms
(1) contains 7 protons;
(2) has an electronic configuration 2, 7? - Write down the formula of the compound formed by atoms X and Y.
उत्तर
(i) As Z has atomic number of 7, hence it has 7 protons. Electronic configuration of 2, 7 means the atom has 2 + 7 = 9 electrons. Therefore, element W has electronic configuration 2, 7 since its atomic number is 9.
(ii) Number of electron in X is 3. Therefore, electronic configuration = (2, 1). As X will try to lose 1 electron to attain stable state, hence, X has valency [1+]
Number of electron in Y is 8. Therefore, electronic configuration = (2, 6). As Y will try to gain 2 electrons to attain stable state, hence, Y has valency [2−]
Since the valency of X is 1+ and valency of Y is 2−
Formula of the compound:
X1+ Y2−
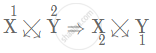
So, we get the formula as X2Y.
APPEARS IN
संबंधित प्रश्न
Complete the statement by filling the gaps using appropriate term from the terms given in the bracket.
(slow, coloured, arrow, fast, smell, milky, physical, product, chemical, reactant, covalent, ionic, octet, duplet, exchange, sharing, equality sign)
Sodium chloride is ______ compound while hydrogen chloride is ______ compound.
What do you understand by redox reactions? Explain oxidation and reduction in terms of loss or gain of electrons.
Name an element which does not contain neutron.
Name the following:
An element having valency 'zero'
How do atoms attain noble gas configurations?
State the type of bonding in the following molecules.
Hydrogen chloride
Draw the electron distribution diagram for the formation of Carbon di oxide (CO2 ) molecule.
______ is the only noble gas that does not have eight electrons in its valence shell.
Match the following:
| 1. | Monoatomic gaseous atom | a. | Electrovalent bond |
| 2. | Octet rule | b. | Benzene |
| 3. | Ionic bond | c. | Water |
| 4. | Non-polar solvent | d. | Electronic theory of valence |
| 5. | Polar solvent | e. | Noble gases |
Write the basic concept of Kossel – Lewis theory.
