Advertisements
Advertisements
Question
Draw the geometric atomic structure of the following atom showing the number of electrons, protons and neutrons in them:
\[\ce{^23_11Na}\]
Solution
Geometric atomic structure of:
\[\ce{^23_11Na}\]
Number of protons p = 11 = e
A = p + n
23 = 11 + n
∴ Number of neutrons n = 12
Electronic configuration 2, 8, 1
K L M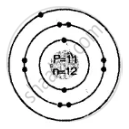
APPEARS IN
RELATED QUESTIONS
What type of bond is formed between two atoms, when the electronegative difference between them is low ?
State the type of bonding in the following molecule.
Calcium oxide
How are X-rays produced?
Name the following:
An element having valency 'zero'
Name the following:
The shell closest to the nucleus of an atom
What do you understand by redox reactions?
Potassium (at No.19) and chlorine (at No.17) react to form a compound. Explain the formation of the compound on the basis of reducing agent.
How do atoms attain Noble gas electronic configuration?
Spot the error/correct the wrong statement:
In the formation of compounds, the inner shell electrons of an atom involved in bonding.
How many electrons are required or released by each atom in water molecule to attain the nearest noble gas configuration?
