Advertisements
Advertisements
Question
Write down the electronic configuration of the following
- 2713X,
- 3517Y.
Write down the number of electrons in X and neutrons in Y and the formula of the compound formed by X and Y.[XY3]
Solution
Electronic configuration of:
(1) 2713X number of electrons = 13
Distribution of elecrtrons in different ordits
13 = [2, 8, 3]
K L M
Number of neutrons in Y
= A − Z = 35 − 17 = 18
(2) 3517Y number of electrons = 17
Distribution of electrons in different orbits
17 = [2, 8, 7]
K L M
Formula of compound formed is
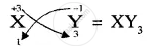
APPEARS IN
RELATED QUESTIONS
Fill in the blank of the following statement :
Cathode rays are a beam of fast moving _________.
Write down the electronic configuration of the following:
(a) `""_13^27"Y"`
(b) `""_17^35"Y"`
Element X has electronic configurations 2, 8, 18, 8, 1. Without identifying X,
- Predict the sign and charge on a simple ion of X.
- Write if X will be an oxidizing agent or a reducing agent. Why?
The electronic configuration of fluoride ion is the same as that of the neon atom. What is the difference between the two?
What is the term defined below?
A bond formed by a shared pair of electrons, each bonding atom contributing one electron to the pair.
Cathode rays are made up of
_______ is a negatively charged particle.
The number of electrons in an element X is 15 and the number of neutrons is 16. Which of the following is the correct representation of the element?
All non–metals will have ______ electrons in the outermost orbit of their atoms.
Helium has two electrons in the outermost orbit and so it is chemically inert.
