Advertisements
Advertisements
प्रश्न
Draw the structures of BCl3.NH3 and AlCl3 (dimer).
उत्तर
In BCl3, the central B atom has six electrons in the valence shell. It is, therefore, an electron-deficient molecule and needs two more electrons to ‘ complete its octet. In other words, BCl3 acts as a Lewis acid. NH3, on the other hand, has a lone pair of electrons which it can donate easily. Therefore, NH3 acts as a Lewis base. The Lewis acid (BC13) and the Lewis base (NH3) combine together to form an adduct as shown below:
\[\begin{array}{cc}
\phantom{....}\ce{NH3}\\
\phantom{}↓\\
\phantom{.}\ce{B}\\
\phantom{.}/\phantom{...}|\phantom{...}\backslash\phantom{}\\
\phantom{}\ce{Cl}\phantom{}\ce{\underset{BCl3.NH3}{Cl}}\phantom{}\ce{Cl}\phantom{}\\
\end{array}\]
In AlC13, Al has six electrons in the valence shell. Therefore, it is an electron-deficient molecule and needs two more electrons to complete its octet. Chlorine, on the other hand, has three lone pairs of electrons. Therefore, to complete its octet, the central Al atom of one molecule accepts a lone pair of electrons from Cl atom of the other molecule forming a dimeric structure as shown below.
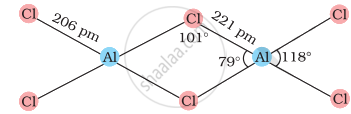
AlCl3 (dimer)
APPEARS IN
संबंधित प्रश्न
Explain what happens when boric acid is heated.
An aqueous solution of borax is _______.
Explain the nature of boric acid as a Lewis acid in water.
When aqueous solution of borax is acidified with hydrochloric acid, a white crystalline solid is formed which is soapy to touch. Is this solid acidic or basic in nature? Explain.
Which of the following is a FALSE statement about boric acid, \[\ce{H3BO3}\]?
Aqueous solution of which of the following boron compounds will be strongly basic in nature?
Diborane (B2H6) reacts independently with O2 and H2O to produce, respectively:
When aqueous solution of AICl3 is concentrated, it furnishes crystals of ______.
Boric acid (H3BO3) is ______.
The reaction of H3N3B3Cl3(A) with LiBH4 in tetrahydrofuran gives inorganic benzene (B). Further, the reaction of (A) with (C) leads to H3N3B3(Me)3. Compounds (B) and (C) respectively, are ______.
