Advertisements
Advertisements
प्रश्न
Draw the structures of BCl3.NH3 and AlCl3 (dimer).
उत्तर
In BCl3, the central B atom has six electrons in the valence shell. It is, therefore, an electron-deficient molecule and needs two more electrons to ‘ complete its octet. In other words, BCl3 acts as a Lewis acid. NH3, on the other hand, has a lone pair of electrons which it can donate easily. Therefore, NH3 acts as a Lewis base. The Lewis acid (BC13) and the Lewis base (NH3) combine together to form an adduct as shown below:
\[\begin{array}{cc}
\phantom{....}\ce{NH3}\\
\phantom{}↓\\
\phantom{.}\ce{B}\\
\phantom{.}/\phantom{...}|\phantom{...}\backslash\phantom{}\\
\phantom{}\ce{Cl}\phantom{}\ce{\underset{BCl3.NH3}{Cl}}\phantom{}\ce{Cl}\phantom{}\\
\end{array}\]
In AlC13, Al has six electrons in the valence shell. Therefore, it is an electron-deficient molecule and needs two more electrons to complete its octet. Chlorine, on the other hand, has three lone pairs of electrons. Therefore, to complete its octet, the central Al atom of one molecule accepts a lone pair of electrons from Cl atom of the other molecule forming a dimeric structure as shown below.
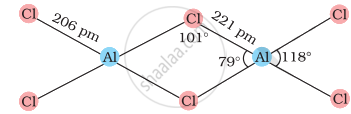
AlCl3 (dimer)
APPEARS IN
संबंधित प्रश्न
Explain what happens when boric acid is heated.
Explain the following reaction.
Hydrated alumina is treated with aqueous NaOH solution.
A certain salt X, gives the following results.
- Its aqueous solution is alkaline to litmus.
- It swells up to a glassy material Y on strong heating.
- When conc. H2SO4 is added to a hot solution of X, white crystal of an acid Z separates out.
Write equations for all the above reactions and identify X, Y and Z.
Write a balanced equation for NaH + B2H6 → ?
What are boranes? Give chemical equation for the preparation of diborane.
Which of the following is a FALSE statement about boric acid, \[\ce{H3BO3}\]?
Which of the following compounds are formed when boron trichloride is treated with water?
In diborane ______.
On the addition of mineral acid to an aqueous solution of borax, the compound formed is ______.
Borazine, also known as inorganic benzene, can be prepared by the reaction of 3-equivalents of “X” with 6-equivalents of “Y”. “X” and “Y”, respectively are ______.
