Advertisements
Advertisements
प्रश्न
Explain the following with an example.
Williamson ether synthesis
उत्तर
It is an important laboratory method for the preparation of symmetrical and unsymmetrical ethers. In this method, an alkyl halide is allowed to react with sodium alkoxide.
\[\ce{R - X + R' - \overset{\overline{\bullet\bullet}}{\underset{\bullet\bullet}{O}}N\overset{+}{a} -> R - \overset{\bullet\bullet}{\underset{\bullet\bullet}{O}} - R' + NaX}\]
Ethers containing substituted alkyl groups (secondary or tertiary) may also be prepared by this method. The reaction involves SN2 attack of an alkoxide ion on primary alkyl halide.
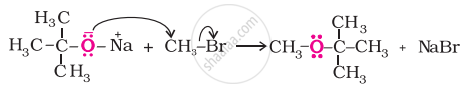
Better results are obtained if the alkyl halide is primary. In case of secondary and tertiary alkyl halides, elimination competes over substitution. If a tertiary alkyl halide is used, an alkene is the only reaction product and no ether is formed. For example, the reaction of CH3ONa with (CH3)3C–Br gives exclusively 2-methylpropene.
\[\begin{array}{cc}
\ce{CH3}\phantom{.................................................}\\
|\phantom{....................................................}\\
\ce{CH3 - C - Br + N\overset{+}{a} \overset{\overline{\bullet\bullet}}{\underset{\bullet\bullet}{O}} - CH3 -> CH3 - C = CH2 + NaBr + CH3OH}\\
\phantom{}|\phantom{.................................}|\phantom{...................}\\
\phantom{}\ce{CH3}\phantom{..........................}\ce{\underset{2-Methylpropene}{CH3}}\phantom{............}
\end{array}\]
APPEARS IN
संबंधित प्रश्न
How do you convert the following :
Phenol to anisole
Write the equations involved in the following reactions : Williamson synthesis
Write the name of the reagent and the equation for the preparation of the following ether by Williamson’s synthesis:
1-Propoxypropane
Preparation of ethers by acid dehydration of secondary or tertiary alcohols is not a suitable method. Give reason.
Dehydration of alcohol to ethers is catalysed by:
\[\ce{(CH3)3CONa + CH3CH2Cl ->[-NaCl] (CH3)3COC2H5}\] is called:
Identify the following named reaction
\[\ce{C2H5Br ->[NaOCH3] C2H5OCH3}\]
Which of the following compounds gives a secondary alcohol upon reaction with methylmagnesium bromide?
Write the mechanism of the following reaction:
\[\ce{2CH3CH2OH ->[H^+][413 K] CH3-CH2-O-CH2-CH3 + H2O}\]
Write the name of the reaction, structure and IUPAC name of the product formed when:
\[\ce{CH3CH2CH(CH3)CH(CH3) ONa}\] reacts with \[\ce{C2H5Br}\]
Write the name of reagent and equation for the preparation of the following ether by Williamson’s synthesis:
2-Methoxy-2-methylpropane
Write the name of reagents and equations for the preparation of the following ethers by Williamson’s synthesis:
2-Methoxy-2-methylpropane
Write the name of reagent and equation for the preparation of the following ethers by Williamson’s synthesis:
2-Methoxy-2-methylpropane
Identify the product (s) is/are formed when 1-methoxy propane is heated with excess HI. Name the mechanism involved in the reaction.
Write the name of reagent and equation for the preparation of the following ethers by Williamson’s synthesis:
2-Methoxy-2-methylpropane
Write the name of reagent and equation for the preparation of the following ethers by Williamson’s synthesis:
2-Methoxy-2-methylpropane
Write the name of reagent and equation for the preparation of the following ether by Williamson’s synthesis:
2-Methoxy-2-methylpropane
Give the structure and IUPAC name of the metamers of 2-methoxy propane.
