Advertisements
Advertisements
प्रश्न
Explain why propanol has higher boiling point than that of the hydrocarbon, butane?
उत्तर
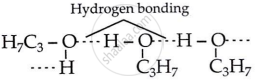
Propanol and butane have nearly the same molecular mass, but propanol has a higher boiling point because of intermolecular hydrogen bonding between its molecules. In butane, hydrogen bonding is not present due to the absence of the polar −OH group. The molecules of butane are connected by weaker van der Waals attraction forces.
APPEARS IN
संबंधित प्रश्न
Give reasons for the following : Boiling point of ethanol is higher in comparison to methoxymethane.
Arrange the following compounds in the increasing order of their acid strength:
p-cresol, p-nitrophenol, phenol
Account for the following:
CH3CHO is more reactive than CH3COCH3 towards reaction with HCN.
Write a chemical reaction to show that the open structure of D-glucose contains the following :
Five alcohol groups
Alcohols have high boiling points because of ____________.
Isopropyl alcohol is obtained by reacting which of the following alkenes with concentrated H2SO4 followed by boiling with H2O?
The correct order of boiling points for primary (1°), secondary (2°) and tertiary alcohol (3°) is:
Alcohols of low molecular weight are _____________.
Which statement is not correct about alcohol?
Explain why alcohols and ethers of comparable molecular mass have different boiling points?
Assertion: Boiling points of alcohols and ethers are high.
Reason: They can form intermolecular hydrogen-bonding.
A solution of phenol in chloroform when treated with aqueous NaOH gives compound P as a major product. The mass percentage of carbon in P is ______. (to the nearest integer) (Atomic mass: C = 12; H = 1; O = 16)
Assertion (A): Alcohols react both as nucleophiles and electrophiles.
Reason (R): The bond between C–O is broken when alcohols react as nucleophiles.
Select the most appropriate answer from the options given below:
How are the following conversions carried out?
Methyl magnesium bromide → 2 -Methylpropan-2-ol.
How is the following conversion carried out?
\[\ce{Methyl magnesium bromide ->2-Methylpropan-2-ol}\]
How is the following conversions carried out?
\[\ce{Methyl magnesium bromide → 2-Methylpropan-2-ol}\].
