Advertisements
Advertisements
प्रश्न
Give a simple chemical test to distinguish between the following pair of compounds:
Pentan-2-one and Pentan-3-one
उत्तर
Pentan-2-one and pentan-3-one can be distinguished by the iodoform test.
Iodoform test: Pentan-2-one is a methyl ketone, so it responds to the iodoform test. However, pentan-3-one is not a methyl ketone and does not respond to this test.
\[\begin{array}{cc}
\ce{O}\phantom{.................................................}\\
||\phantom{.................................................}\\
\ce{\underset{Pentan-2-one}{CH3CH2CH2 - C - CH3} + 3NaOI ->\underset{Sodium butanoate}{CH3CH2CH2COONa} + \underset{(yellow ppt)}{\underset{Iodoform}{CHI3\downarrow}} + 2NaOH}\
\end{array}\]
\[\begin{array}{cc}
\ce{O}\phantom{.......................................}\\
||\phantom{.......................................}\\
\ce{\underset{Pentan-3-one}{CH3CH2 - C - CH2CH3} + NaOI -> No yellow ppt of iodoform}\
\end{array}\]
APPEARS IN
संबंधित प्रश्न
Give a simple chemical test to distinguish between the following pair of compounds:
Ethanal and Propanal
Give a simple chemical test to distinguish between the following pair of compounds :
CH3CH2CHO and CH3CH2COCH3
Propanal and Propanone
Out of CH3CH2 – CO – CH2 – CH3 and CH3CH2 – CH2 – CO – CH3, which gives iodoform test?
Give a simple chemical test to distinguish between the following pair of compounds:
Phenol and Benzoic acid
Give a simple chemical test to distinguish between the following pair of compounds:
Benzoic acid and Ethyl benzoate
An organic compound contains 69.77% carbon, 11.63% hydrogen and rest oxygen. The molecular mass of the compound is 86. It does not reduce Tollens’ reagent but forms an addition compound with sodium hydrogensulphite and give positive iodoform test. On vigorous oxidation it gives ethanoic and propanoic acid. Write the possible structure of the compound.
Which of the following compounds will give butanone on oxidation with alkaline \[\ce{KMnO4}\] solution?
Oxidation of ketones involves carbon-carbon bond cleavage. Name the products formed on oxidation of 2, 5-dimethylhexan-3-one.
Which sugar does not reduce Fehling's solution?
Solvent used for dewaxing of petroleum products are
Acetaldehyde cannot show?
A hydrocarbon (A) with molecular formula C5H10 on ozonolysis gives two products (B) and (C). Both (B) and (C) give a yellow precipitate when heated with iodine in presence of NaOH while only (B) give a silver mirror on reaction with Tollen’s reagent.
- Identify (A), (B) and (C).
- Write the reaction of B with Tollen’s reagent.
- Write the equation for iodoform test for C.
- Write down the equation for aldol condensation reaction of B and C.
Write chemical test to distinguish between the following compounds:
Phenol and Benzoic acid
The major products formed in the following reaction sequence A and B are:
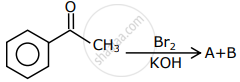
An organic compound neither reacts with neutral ferric chloride solution nor with Fehling solution. It however, reacts with Grignard reagent and gives positive iodoform test. The compound is:
The correct set of products obtained in the following reactions:
- \[\ce{RCN ->[reduction]}\]
- \[\ce{RCN ->[(i) CH3MgBr][(ii) H2O]}\]
- \[\ce{RNC ->[hydrolysis]}\]
- \[\ce{RNH2 ->[HNO2]}\]
Choose the reaction which is not possible:
