Advertisements
Advertisements
प्रश्न
Give a simple chemical test to distinguish between the following pair of compounds:
Benzaldehyde and Acetophenone
उत्तर
Tollen’s Test: Aldehydes respond to Tollen’s test. Benzaldehyde, being an aldehyde, reduces Tollen’s reagent to give a red-brown precipitate of Cu2O, but acetophenone, being a ketone, does not.
\[\ce{\underset{acetophenone}{C6H5COCH3} + \underset{Hypoiodite}{\underset{Sodium}{3NaOI}}->\underset{Benzoate}{\underset{Sodium}{C6H5COONa}} + \underset{(Yellow ppt)}{\underset{Iodoform}{CHI3}} + 2NaOH}\]
Iodoform test: Acetophenone, being a methyl ketone, undergoes oxidation by sodium hypoiodite (NaOI) to give a yellow ppt. of iodoform. However, benzaldehyde does not respond to this test.
\[\ce{\underset{Acetophenone}{C6H5COCH3} + \underset{hypoiodite}{\underset{Sodium}{3NaOI}}->\underset{benzoate}{\underset{Sodium}{C6H5COONa}} + \underset{(yellow ppt)}{\underset{iodoform}{CHI3}} + 2NaOH}\]
\[\ce{\underset{Benzaldehyde}{C6H5CHO} + NaOI -> No yellow ppt of CHI3}\]
APPEARS IN
संबंधित प्रश्न
Give a simple chemical test to distinguish between the following pair of compounds:
Ethanal and Propanal
Distinguish between:
C6H5-COCH3 and C6H5-CHO
Distinguish between: CH3COOH and HCOOH
Propanal and Propanone
Give a simple chemical test to distinguish between the following pair of compounds:
Pentan-2-one and Pentan-3-one
Complete the synthesis by giving missing starting material, reagent or product.
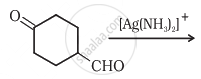
Which of the following compounds will give butanone on oxidation with alkaline \[\ce{KMnO4}\] solution?
Solvent used for dewaxing of petroleum products are
Acetone and acetaldehyde are differentiated by
Fehilng's test is positive for
A hydrocarbon (A) with molecular formula C5H10 on ozonolysis gives two products (B) and (C). Both (B) and (C) give a yellow precipitate when heated with iodine in presence of NaOH while only (B) give a silver mirror on reaction with Tollen’s reagent.
- Identify (A), (B) and (C).
- Write the reaction of B with Tollen’s reagent.
- Write the equation for iodoform test for C.
- Write down the equation for aldol condensation reaction of B and C.
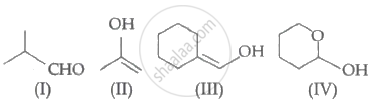
Which among the above compound/s does/do not form Silver mirror when treated with Tollen's reagent?
The correct set of products obtained in the following reactions:
- \[\ce{RCN ->[reduction]}\]
- \[\ce{RCN ->[(i) CH3MgBr][(ii) H2O]}\]
- \[\ce{RNC ->[hydrolysis]}\]
- \[\ce{RNH2 ->[HNO2]}\]
In Tollen's test for aldehyde, the overall number of electrons(s) transferred to the Tollen's reagent formula \[\ce{[Ag(NH3)2]+}\] per aldehyde group to form silver mirror is ______. (Round off to the nearest integer)
The reagent that can be used to distinguish acetophenone and benzophenone is ______.
