Advertisements
Advertisements
Question
Give a simple chemical test to distinguish between the following pair of compounds:
Benzaldehyde and Acetophenone
Solution
Tollen’s Test: Aldehydes respond to Tollen’s test. Benzaldehyde, being an aldehyde, reduces Tollen’s reagent to give a red-brown precipitate of Cu2O, but acetophenone, being a ketone, does not.
\[\ce{\underset{acetophenone}{C6H5COCH3} + \underset{Hypoiodite}{\underset{Sodium}{3NaOI}}->\underset{Benzoate}{\underset{Sodium}{C6H5COONa}} + \underset{(Yellow ppt)}{\underset{Iodoform}{CHI3}} + 2NaOH}\]
Iodoform test: Acetophenone, being a methyl ketone, undergoes oxidation by sodium hypoiodite (NaOI) to give a yellow ppt. of iodoform. However, benzaldehyde does not respond to this test.
\[\ce{\underset{Acetophenone}{C6H5COCH3} + \underset{hypoiodite}{\underset{Sodium}{3NaOI}}->\underset{benzoate}{\underset{Sodium}{C6H5COONa}} + \underset{(yellow ppt)}{\underset{iodoform}{CHI3}} + 2NaOH}\]
\[\ce{\underset{Benzaldehyde}{C6H5CHO} + NaOI -> No yellow ppt of CHI3}\]
APPEARS IN
RELATED QUESTIONS
Predict the products of the following reactions :
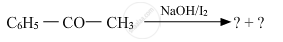
Give a simple chemical test to distinguish between the following pair of compounds :
CH3CH2CHO and CH3CH2COCH3
Propanal and Propanone
Out of CH3CH2 – CO – CH2 – CH3 and CH3CH2 – CH2 – CO – CH3, which gives iodoform test?
Give a simple chemical test to distinguish between the following pair of compounds:
Acetophenone and Benzophenone
Give a simple chemical test to distinguish between the following pair of compounds:
Phenol and Benzoic acid
Which of the following compounds will give butanone on oxidation with alkaline \[\ce{KMnO4}\] solution?
Solvent used for dewaxing of petroleum products are
Fehilng's test is positive for
Which of the following tests/reactions is given by aldehydes as well as ketones?
Write chemical test to distinguish between the following compounds:
Phenol and Benzoic acid
What is the composition of Fehling's reagent?
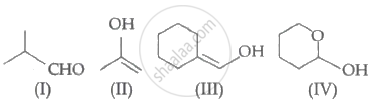
Which among the above compound/s does/do not form Silver mirror when treated with Tollen's reagent?
An organic compound neither reacts with neutral ferric chloride solution nor with Fehling solution. It however, reacts with Grignard reagent and gives positive iodoform test. The compound is:
