Advertisements
Advertisements
Question
Propanal and Propanone
Solution 1
Propanal and propanone can be distinguished by the following tests:
- Tollen’s test: Propanal is an aldehyde. Thus, it reduces Tollen’s reagent. But, propanone being a ketone does not reduce Tollen’s reagent.
\[\ce{\underset{Propanal}{CH3CH2CHO} + \underset{Tollen's reagent}{2[Ag(NH3)2]+} + 3OH- -> \underset{Propanoate ion}{CH3CH2COO-} + \underset{Silver mirror}{Ag\downarrow} + NH3 + 2H2O}\] - Fehling’s test: Aldehydes respond to Fehling’s test, but ketones do not. Propanal being an aldehyde reduces Fehling’s solution to a red-brown precipitate of Cu2O, but propanone being a ketone does not.
\[\ce{\underset{Propanal}{CH3CH2CHO} + 2Cu^2+ + 5OH- -> \underset{Propanaoate ion}{CH3CH2COO-} + \underset{(Red-brown ppt)}{\underset{Cuprous oxide}{Cu2O\downarrow}}+ 2H2O}\] - Iodoform test: Aldehydes and ketones with at least one methyl group linked to the carbonyl carbon atom respond to the iodoform test. They are oxidized by sodium hypoiodite (NaOI) to give iodoforms. Propanone being a methyl ketone responds to this test, but propanal does not.
\[\ce{\underset{Propanone}{CH3COCH3} + 3NaOI -> \underset{Sodium acetate}{CH3COONa} + \underset{(yellow ppt)}{\underset{Iodoform}{CHI3}}+ 2NaOH}\]
Solution 2
| Reagent | Propanal | Propanone |
| Tollen’s Reagent | On heating with Tollen’s reagent, a silver mirror is formed on the inner walls of the test tube. Aldehyde groups reduce the Tollen's reagent. | No change in propanone. |
APPEARS IN
RELATED QUESTIONS
Distinguish between: CH3COOH and HCOOH
A and B are two functional isomers of compound C3H6O.On heating with NaOH and I2, isomer B forms yellow precipitate of iodoform whereas isomer A does not form any precipitate. Write the formulae of A and B.
Give a simple chemical test to distinguish between the following pair of compounds :
CH3CH2CHO and CH3CH2COCH3
Give a simple chemical test to distinguish between the following pair of compounds:
Benzoic acid and Ethyl benzoate
Give a simple chemical test to distinguish between the following pair of compounds:
Pentan-2-one and Pentan-3-one
Give a simple chemical test to distinguish between the following pair of compounds:
Benzaldehyde and Acetophenone
Complete the synthesis by giving missing starting material, reagent or product.
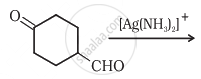
Oxidation of ketones involves carbon-carbon bond cleavage. Name the products formed on oxidation of 2, 5-dimethylhexan-3-one.
Which of the following compounds gives a positive Tollen's test but negative Fehling's test?
Which sugar does not reduce Fehling's solution?
Acetaldehyde cannot show?
Write chemical test to distinguish between the following compounds:
Phenol and Benzoic acid
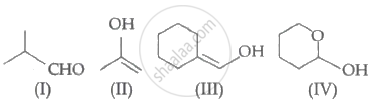
Which among the above compound/s does/do not form Silver mirror when treated with Tollen's reagent?
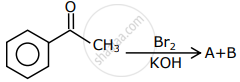
The major products formed in the following reaction sequence A and B are:
The reagent that can be used to distinguish acetophenone and benzophenone is ______.
An organic compound 'A' with the molecular formula C4H8O2 undergoes acid hydrolysis to form two compounds 'B' and 'C'. Oxidation of 'C' with acidified potassium permanganate also produces 'B'. Sodium salt of 'B' on heating with soda lime gives methane.
- Identify 'A', 'B' and 'C'.
- Out of 'B' and 'C', which will have higher boiling point? Give reason.
