Advertisements
Advertisements
Question
The reagent that can be used to distinguish acetophenone and benzophenone is ______.
Options
2, 4-dinitrophenyl hydrazine
aqueous NaHSO3
Fehling solution
I2 and NaOH
Solution
The reagent that can be used to distinguish acetophenone and benzophenone is I2 and NaOH.
Explanation:
In the preceding test, the I2 and NaOH tests are utilized to differentiate between acetophenone and benzophenone. When iodine molecules react with acetophenone, they generate methyl iodide by combining with methyl groups. The synthesis of methyl iodide produces a yellow precipitate, indicating that the molecule is acetophenone.
However, no precipitate is generated when I2 and NaOH combine with benzophenone. Because benzophenone has a ring that resists precipitation, this reaction does not occur.
APPEARS IN
RELATED QUESTIONS
Distinguish between:
C6H5-COCH3 and C6H5-CHO
Out of CH3CH2 – CO – CH2 – CH3 and CH3CH2 – CH2 – CO – CH3, which gives iodoform test?
Which of the following compounds will give butanone on oxidation with alkaline \[\ce{KMnO4}\] solution?
Fehilng's test is positive for
The major products formed in the following reaction sequence A and B are:
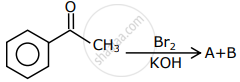
The correct set of products obtained in the following reactions:
- \[\ce{RCN ->[reduction]}\]
- \[\ce{RCN ->[(i) CH3MgBr][(ii) H2O]}\]
- \[\ce{RNC ->[hydrolysis]}\]
- \[\ce{RNH2 ->[HNO2]}\]
Choose the reaction which is not possible:
In Tollen's test for aldehyde, the overall number of electrons(s) transferred to the Tollen's reagent formula \[\ce{[Ag(NH3)2]+}\] per aldehyde group to form silver mirror is ______. (Round off to the nearest integer)
An organic compound 'A' with molecular formula C5H8O2 is reduced to n-pentane with hydrazine followed by heating with NaOH and glycol. 'A' forms a dioxime with hydroxylamine and gives a positive iodoform and Tollen's test. Identify 'A' and give its reaction for iodoform and Tollen's test.
You are given four organic compounds “A”, “B” , “C” and “D”. The compounds “A”, “B” and “C” form an orange-red precipitate with 2, 4 DNP reagent. Compounds “A” and “B” reduce Tollen’s reagent while compounds “C” and “D” do not. Both “B” and “C” give a yellow precipitate when heated with iodine in the presence of NaOH. Compound “D” gives brisk effervescence with sodium bicarbonate solution. Identify “A”, “B”, “C” and “D” given the number of carbon atoms in three of these carbon compounds is three while one has two carbon atoms. Give an explanation for our answer.
