Advertisements
Advertisements
Question
Out of CH3CH2 – CO – CH2 – CH3 and CH3CH2 – CH2 – CO – CH3, which gives iodoform test?
Solution 1
CH3CH2 – CH2 –CO –CH3
Solution 2
Pentan-2-one (CH3-CH2-CH2-CO-CH3) give yellow precipitate of CHI3 with NaOI, thats means it gives iodoform test.

Pentan-3-one (CH3-CH2-CO-CH2-CH3) does not give yellow precipitate with CHI3 with NaOI, so Pentan-3-one does not give iodoform test.
APPEARS IN
RELATED QUESTIONS
Distinguish between: CH3COOH and HCOOH
A and B are two functional isomers of compound C3H6O.On heating with NaOH and I2, isomer B forms yellow precipitate of iodoform whereas isomer A does not form any precipitate. Write the formulae of A and B.
Propanal and Propanone
Which of the following compounds will give butanone on oxidation with alkaline \[\ce{KMnO4}\] solution?
Oxidation of ketones involves carbon-carbon bond cleavage. Name the products formed on oxidation of 2, 5-dimethylhexan-3-one.
Acetaldehyde cannot show?
What is the composition of Fehling's reagent?
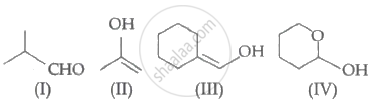
Which among the above compound/s does/do not form Silver mirror when treated with Tollen's reagent?
The reagent that can be used to distinguish acetophenone and benzophenone is ______.
An organic compound 'A' with the molecular formula C4H8O2 undergoes acid hydrolysis to form two compounds 'B' and 'C'. Oxidation of 'C' with acidified potassium permanganate also produces 'B'. Sodium salt of 'B' on heating with soda lime gives methane.
- Identify 'A', 'B' and 'C'.
- Out of 'B' and 'C', which will have higher boiling point? Give reason.
