Commerce (English Medium)
Science (English Medium)
Arts (English Medium)
Academic Year: 2013-2014
Date: March 2014
Advertisements
Give one example each of 'oil in water' and 'water in oil' emulsion.
Chapter: [0.05] Surface Chemistry
Which reducing agent is employed to get copper from the leached low-grade copper ore?
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Which of the following is a more stable complex and why ?
Chapter: [0.05] Coordination Compounds
Write the IUPAC name of the compound.
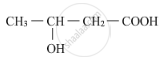
Chapter: [0.05] Coordination Compounds
Which of the following isomers is more volatile:
o-nitrophenol or p-nitrophenol?
Chapter: [0.05] Coordination Compounds
What are isotonic solutions? Explain with one example.
Chapter: [0.01] Solutions
Arrange the following.
In increasing order of solubility in water, C6H5NH2, (C2H5)2NH, C2H5NH2
Chapter: [0.09] Amines
Which of the two components of starch is water soluble?
Chapter: [0.01] Solutions
An element with density 11.2 g cm–3 forms a f.c.c. lattice with edge length of 4 × 10–8 cm.
Calculate the atomic mass of the element.
(Given : NA = 6.022 × 1023 mol–1)
Chapter: [0.01] Solid State
Examine the given defective crystal:

Answer the following questions :
(i) What type of stoichiometric defect is shown by the crystal?
(ii) How is the density of the crystal affected by this defect?
(iii) What type of ionic substances show such defect?
Chapter: [0.01] Solid State
Calculate the mass of a compound (molar mass = 256 g mol−1) to be dissolved in 75 g of benzene to lower its freezing point by 0.48 K (Kf = 5.12 K kg mol−1).
Chapter: [0.01] Solutions
Define an ideal solution and write one of its characteristics.
Chapter: [0.01] Solutions
Write two differences between 'order of reaction' and 'molecularity of reaction'.
Chapter: [0.03] Chemical Kinetics
Outline the principles behind the refining of metals by the following methods:
Zone refining method
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Outline the principles behind the refining of metals by the following methods:
Chromatographic method
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Complete the following chemical equations :
Ca3P2 + H2O →
Chapter: [0.07] P - Block Elements
Complete the following chemical equations :
Cu + H2SO4(conc.) →
Chapter: [0.04] d-block and f-block Elements
Write the IUPAC name of the complex [Cr(NH3)4Cl2]+. What type of isomerism does it exhibit?
Chapter: [0.05] Coordination Compounds
Which alkyl halide from the following pair is chiral and undergoes faster SN2 reaction?
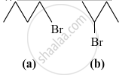
Chapter: [0.09] Amines
Out of SN1 and SN2, which reaction occurs with
(a) Inversion of configuration
(b) Racemisation
Chapter: [0.09] Amines
Draw the structure of major monohalo product in each of the following reactions :
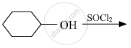
Chapter: [0.06] Haloalkanes and Haloarenes
Draw the structure of major monohalo product in each of the following reactions :
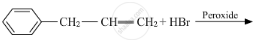
Chapter:
In reference to Freundlich adsorption isotherm, write the expression for adsorption of gases on solids in the form of an equation.
Chapter: [0.05] Surface Chemistry
Write an important characteristic of lyophilic sols.
Chapter: [0.05] Surface Chemistry
Based on the type of particles of dispersed phase, give one example each of associated colloid and multimolecular colloid.
Chapter: [0.05] Surface Chemistry
Advertisements
Write the structures of the following molecules: XeOF4
Chapter: [0.07] P - Block Elements
Draw the structure of the following:
H2SO4
Chapter: [0.07] P - Block Elements
Write the structural difference between white phosphorus and red phosphorus.
Chapter: [0.07] P - Block Elements
Account for the following:
PCl5 is more covalent than PCl3.
Chapter: [0.01] Solid State
Account for the following :
Iron on reaction with HCl forms FeCl2 nd not FeCl3.
Chapter: [0.06] General Principles and Processes of Isolation of Elements
The two O−O bond lengths in the ozone molecule are equal.
Chapter: [0.07] P - Block Elements
The following data were obtained during the first order thermal decomposition of SO2Cl2 at a constant volume :
SO2Cl2 (g) → SO2 (g) + Cl2 (g)
| Experiment | Time/s–1 | Total pressure/atm |
| 1 | 0 | 0.4 |
| 2 | 100 | 0.7 |
Calculate the rate constant.
(Given : log 4 = 0.6021, log 2 = 0.3010)
Chapter: [0.03] Chemical Kinetics
Give two examples of macromolecules that are chosen as drug targets
Chapter: [0.16] Chemistry in Everyday Life
Explain the term Antiseptics
Chapter: [0.16] Chemistry in Everyday Life
Why is use of aspartame limited to cold foods and drinks?
Chapter: [0.16] Chemistry in Everyday Life
Deficiency of which vitamin causes night-blindness?
Chapter:
Name the base that is found in nucleotide of RNA only.
Chapter: [0.1] Biomolecules
Glucose on reaction with HI gives n-hexane. What does it suggest about the structure of glucose?
Chapter: [0.1] Biomolecules
After the ban on plastic bags, students of a school decided to make people aware of the harmful effects of plastic bags on the environment and Yamuna River. To make the awareness more impactful, they organised a rally by partnering with other schools and distributed paper bags to vegetable vendors, shopkeepers and departmental stores. All the students pledged not to use polythene bags in the future to save the Yamuna River.
After reading the above passage, answer the following questions:
(i) What values are shown by the students?
(ii) What are bio-degradable polymers? Give one example.
(iii) Is polythene a condensation or an addition polymer?
Chapter: [0.15] Polymers
Give the structures of A, B and C in the following reactions :
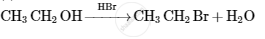
Chapter: [0.09] Amines
Write the equation involved in the following reaction:
Reimer-Tiemann reaction
Chapter: [0.07] Alcohols, Phenols and Ethers
Give the structures of A, B and C in the following reactions :

Chapter: [0.09] Amines
Give the structures of A, B and C in the following reactions :

Chapter: [0.09] Amines
How will you convert the following?
Nitrobenzene into aniline
Chapter: [0.05] Coordination Compounds
How will you convert Ethanoic acid into methanamine
Chapter: [0.09] Amines
How will you convert the following?
Aniline into N−phenylethanamide
Chapter: [0.05] Coordination Compounds
Advertisements
Define the following terms :
Limiting molar conductivity
Chapter: [0.02] Electrochemistry
Define the following term:
Fuel cell
Chapter: [0.02] Electrochemistry
Resistance of a conductivity cell filled with 0.1 mol L−1 KCl solution is 100 Ω. If the resistance of the same cell when filled with 0.02 mol L−1 KCl solution is 520 Ω, calculate the conductivity and molar conductivity of 0.02 mol L−1KCl solution. The conductivity of 0.1 mol L−1 KCl solution is 1.29 × 10−2 Ω−1 cm−1.
Chapter: [0.02] Electrochemistry
State Faraday's first law of electrolysis.
Chapter: [0.02] Electrochemistry
How much charge in terms of Faraday is required for the reduction of 1 mol of Cu2+ to Cu?
Chapter: [0.02] Electrochemistry
Calculate emf of the following cell at 298 K:
Mg(s) | Mg2+(0.1 M) || Cu2+ (0.01) | Cu(s)
[Given Eocell = +2.71 V, 1 F = 96500 C mol–1]
Chapter: [0.02] Electrochemistry
How do you prepare:
K2MnO4 from MnO2?
Chapter: [0.07] P - Block Elements
How do you prepare:
Na2Cr2O7 from Na2CrO4?
Chapter: [0.07] P - Block Elements
Account for the following:
Mn2+ is more stable than Fe2+ towards oxidation to +3 state.
Chapter: [0.04] d-block and f-block Elements
Account for the following
The enthalpy of atomisation is lowest for Zn in 3d series of the transition elements.
Chapter: [0.07] P - Block Elements
How do you prepare:
Actinoid elements show wide range of oxidation states.
Chapter: [0.04] d-block and f-block Elements
Name the elements of 3d transition series that show maximum number of oxidation states. Why does this happen?
Chapter: [0.04] d-block and f-block Elements
Which transition metal of 3d series has positive E° (M2+/M) value and why?
Chapter: [0.04] d-block and f-block Elements
Out of Cr3+ and Mn3+, which is a stronger oxidising agent and why?
Chapter: [0.04] d-block and f-block Elements
Name a member of the lanthanoid series that is well-known to exhibit +2 oxidation state.
Chapter: [0.04] d-block and f-block Elements
Complete the following equation : MnO4- + 8H+ + 5e- →
Chapter: [0.04] d-block and f-block Elements
Write the products of the following reactions:
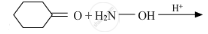
Chapter: [0.1] Biomolecules
Write the products of the following reactions:
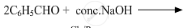
Chapter: [0.1] Biomolecules
Write the products of the following reactions:
![]()
Chapter: [0.1] Biomolecules
Give simple chemical tests to distinguish between the following pair of compounds:
Benzaldehyde and Benzoic acid
Chapter: [0.09] Amines
Propanal and Propanone
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Account for the following:
CH3CHO is more reactive than CH3COCH3 towards reaction with HCN.
Chapter: [0.07] Alcohols, Phenols and Ethers
Account for the following:
Carboxylic acid is a stronger acid than phenol.
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write the equations involved in the following reactions :
Wolff-Kishner reduction
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write the chemical equations to illustrate the following name reactions:
Aldol condensation
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write the chemical equations to illustrate the following name reaction:
Cannizzaro’s reaction
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CBSE previous year question papers Class 12 Chemistry with solutions 2013 - 2014
Previous year Question paper for CBSE Class 12 -2014 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CBSE Class 12.
How CBSE Class 12 Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
