Advertisements
Advertisements
Questions
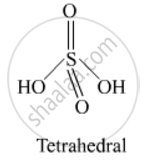
Draw the structure of the following:
H2SO4
Draw the structure of sulphuric acid.
Solution
RELATED QUESTIONS
What is molecular formula of oleum?
(a) H2SO3
(b) H2SO4
(c) H2S2O7
(d) H2S2O8
Write the conditions to maximize the yield of H2SO4 by contact process.
Complete the following equations: CaF2+H2SO4 →
Complete the following equations: C + conc. H2SO4 →
Mention three areas in which H2SO4 plays an important role.
Why is `K_(a_2) "<<" K_(a_1)` for `H_2SO_4` in water?
Describe the manufacture of H2SO4 by contact process?
Write balanced equations for NaCl is heated with sulphuric acid in the presence of MnO2
What is the action of concentrated sulphuric acid on phosphorous pentachloride
What happens when dilute sulphuric acid is treated with Fe
What happens when dilute sulphuric acid is treated with CaF2
What is the action of concentrated sulphuric acid on potassium chlorate?
When concentrated sulphuric acid was added to an unknown salt present in a test tube a brown gas (A) was evolved. This gas intensified when copper turnings were added to this test tube. On cooling, the gas (A) changed into a colourless solid (B).
1) Identify (A) and (B).
2) Write the structures of (A) and (B).
3) Why does gas (A) change to solid on cooling?
Give balanced chemical equations for Sulphuric acid is treated with hydrogen sulphide
Write chemical reactions for different steps in the manufacture of sulphuric acid by lead chamber process. Draw the structure of phosphorous pentachloride
Complete and balance the following equations:
S+H2SO4(conc.) →
Write a balanced chemical equation for the following reaction:
Sulphuric acid is treated with phosphorous.
Write the reaction of conc. \[\ce{H2SO4}\] with sugar.
Which of the following reaction is NOT involved in contact process used for manufacturing sulfuric acid?
Identify the INCORRECT statement about H2SO4.
Identify the products formed when conc. sulfuric acid reacts with calcium fluoride.
The molecular formula of oleum is ____________.
The sulfuric acid obtained by contact process is ____________ % pure.
Which among the following catalysts is used in manufacture of sulphuric acid by contact process?
What is the ratio of volumes of gases involved in the preparation of sulphur trioxide from sulphur dioxide and dioxygen respectively under similar conditions of temperature and pressure?
In the manufacture of sulphuric acid by contact process Tyndall box is used to ____________.
Hot conc. \[\ce{H2SO4}\] acts as the moderately strong oxidising agent. It oxidises both metals and non-metals. Which of the following element is oxidised by conc. \[\ce{H2SO4}\] into two gaseous products?
Which of the following statements are correct?
(i) S – S bond is present in \[\ce{H2S2O6}\].
(ii) In peroxosulphuric acid \[\ce{(H2SO5)}\] sulphur is in +6 oxidation state.
(iii) Iron powder along with \[\ce{Al2O3}\] and \[\ce{K2O}\] is used as a catalyst in the preparation of \[\ce{NH3}\] by Haber’s process.
(iv) Change in enthalpy is positive for the preparation of \[\ce{SO3}\] by catalytic oxidation of \[\ce{SO2}\].
The oxidation number of sulphur in Na2S4O6 is:-
The number of dative bonds in sulphric acid molecule is ______.
What is the catalyst used for the oxidation of SO2 to SO3 in the lead chamber process for the manufacture of sulphuric acid?
