Advertisements
Advertisements
Question
Write chemical reactions for different steps in the manufacture of sulphuric acid by lead chamber process. Draw the structure of phosphorous pentachloride
Solution
2SO2 + O2 +2H2O`overset("No" )(->)`2H2SO4
The above net reaction can be written as the sum of the following reactions taking place in different steps.
1) 2H2O +NO `rightarrow` HNO3 +3H+
2) S + O2 `rightarrow` SO2 (Oxidation of sulphur)
3) 2HNO3 + 2SO2 `rightarrow` H2O +NO +NO2 +2SO3
Nitric acid or NO2 is used to oxidize SO2 to SO3.
Or SO2 +NO2 `rightarrow` SO3 + NO
4) SO3 +H2O `rightarrow` H2SO4
Sulphur trioxide reacts with water to form H2SO4 .
Structure of phosphorous pentachloride :
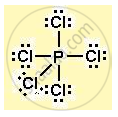
Phosphorous has 5 valence electrons. Each electron is shared with a chlorine atom.
APPEARS IN
RELATED QUESTIONS
What is molecular formula of oleum?
(a) H2SO3
(b) H2SO4
(c) H2S2O7
(d) H2S2O8
Write the conditions to maximize the yield of H2SO4 by contact process.
Complete the following equations: CaF2+H2SO4 →
Complete the following equations: C + conc. H2SO4 →
Why is `K_(a_2) "<<" K_(a_1)` for `H_2SO_4` in water?
What happens when dilute sulphuric acid is treated with Fe
What happens when dilute sulphuric acid is treated with CaF2
What is the action of concentrated sulphuric acid on copper
What is the action of concentrated sulphuric acid on potassium chlorate?
When concentrated sulphuric acid was added to an unknown salt present in a test tube a brown gas (A) was evolved. This gas intensified when copper turnings were added to this test tube. On cooling, the gas (A) changed into a colourless solid (B).
1) Identify (A) and (B).
2) Write the structures of (A) and (B).
3) Why does gas (A) change to solid on cooling?
Give balanced chemical equations for Sulphuric acid is treated with hydrogen sulphide
Complete and balance the following equations:
S+H2SO4(conc.) →
Write structure and molecular formula for the following compounds:
a. Orthophosphoric acid
b. Sulphurous acid
Write the reaction of conc. \[\ce{H2SO4}\] with sugar.
Which of the following reaction is NOT involved in contact process used for manufacturing sulfuric acid?
Gas 'X' is prepared by treating sodium sulfite with dilute sulfuric acid. When gas 'X' is oxidised by dioxygen in presence of vanadium (V) oxide, gas 'Y' is formed. Identify X and Y.
The molecular formula of oleum is ____________.
The sulfuric acid obtained by contact process is ____________ % pure.
Which among the following catalysts is used in manufacture of sulphuric acid by contact process?
What is the ratio of volumes of gases involved in the preparation of sulphur trioxide from sulphur dioxide and dioxygen respectively under similar conditions of temperature and pressure?
Which of the following statements are correct?
(i) S – S bond is present in \[\ce{H2S2O6}\].
(ii) In peroxosulphuric acid \[\ce{(H2SO5)}\] sulphur is in +6 oxidation state.
(iii) Iron powder along with \[\ce{Al2O3}\] and \[\ce{K2O}\] is used as a catalyst in the preparation of \[\ce{NH3}\] by Haber’s process.
(iv) Change in enthalpy is positive for the preparation of \[\ce{SO3}\] by catalytic oxidation of \[\ce{SO2}\].
What is the catalyst used for the oxidation of SO2 to SO3 in the lead chamber process for the manufacture of sulphuric acid?
